Immunotherapy in high-risk chemotherapy-resistant patients with metastatic solid tumors and hematological malignancies using intentionally mismatched donor lymphocytes activated with rIL-2: a phase I study
- PMID: 20563804
- PMCID: PMC11031035
- DOI: 10.1007/s00262-010-0878-1
Immunotherapy in high-risk chemotherapy-resistant patients with metastatic solid tumors and hematological malignancies using intentionally mismatched donor lymphocytes activated with rIL-2: a phase I study
Abstract
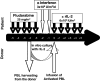
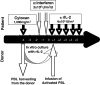
The feasibility and safety of immunotherapy mediated by intentionally mismatched rIL-2 activated killer lymphocytes (IMAK) with no prior stem cell engraftment was investigated in patients with advanced chemotherapy-resistant hematological malignancies and metastatic solid tumors. Our goals were to maximize anti-cancer activity by using intentionally mismatched donor lymphocytes; amplify killing of target cancer cells by rIL-2 activation of killer cells in vitro and in vivo, and avoid the risk of graft-versus-host disease (GVHD) by anticipated rejection of alloreactive donor lymphocytes. Conditioning consisted of 5 days of fludarabine 25 mg/m(2) or a single dose of cyclophosphamide 1,000 mg/m(2), 2 subcutaneous injections of alpha interferon (IFN) 3 x 10(6) and COX2 inhibitors, followed by administration of IMAK (65 +/- 5 CD3(+)CD56(-); 17 +/- 5 CD3(-)CD56(+)) in conjunction with low dose subcutaneous rIL-2 (6 x 10(6) IU/m(2)/day) for 5 days for continuous activation of alloreactive donor lymphocytes prior to their anticipated rejection. Here, we present our phase 1 clinical study data in a cohort of 40 high-risk patients with metastatic solid tumors and hematological malignancies. Treatment was accompanied by some malaise and occasional self-limited fever but otherwise well tolerated on an outpatient basis. Transient engraftment of donor cells was documented in two patients and only one developed self-limited grade 1 GVHD. Among patients with chemotherapy-resistant disease, long-term progression-free survival was recorded in 5 of 21 evaluable patients with metastatic solid tumors and in four of five patients with hematological malignancies. We conclude that the proposed procedure is feasible, safe, and potentially effective, with some otherwise resistant cancer patients long-term disease-free, thus justifying larger Phase II studies in patients with hematological malignancies and metastatic solid tumors, preferably at a stage of minimal residual disease with the goal in mind to eradicate all malignant cells at an early stage of the disease.
Figures
Similar articles
-
Immunotherapy with cure potential of multi-drug resistant hematologic malignancies using IL-2 preactivated intentionally mismatched donor lymphocyte.J Cancer Res Clin Oncol. 2023 Sep;149(11):9277-9284. doi: 10.1007/s00432-023-04780-5. Epub 2023 May 19. J Cancer Res Clin Oncol. 2023. PMID: 37202579 Free PMC article.
-
Interleukin-2 therapy after bone marrow or stem cell transplantation for hematologic malignancies.Cancer J Sci Am. 1997 Dec;3 Suppl 1:S48-53. Cancer J Sci Am. 1997. PMID: 9457394 Clinical Trial.
-
Donor lymphocyte infusion: the use of alloreactive and tumor-reactive lymphocytes for immunotherapy of malignant and nonmalignant diseases in conjunction with allogeneic stem cell transplantation.J Hematother Stem Cell Res. 2002 Apr;11(2):265-76. doi: 10.1089/152581602753658457. J Hematother Stem Cell Res. 2002. PMID: 11983098 Review.
-
Experimental evidence of interleukin-2 activity in bone marrow transplantation.Cancer J Sci Am. 1997 Dec;3 Suppl 1:S37-42. Cancer J Sci Am. 1997. PMID: 9457392 Review.
-
Nonmyeloablative stem cell transplantation: reduced-intensity conditioning for cancer immunotherapy--from bench to patient bedside.Semin Oncol. 2004 Feb;31(1):4-21. doi: 10.1053/j.seminoncol.2003.10.016. Semin Oncol. 2004. PMID: 14970932 Review.
Cited by
-
In vivo proof of concept of adoptive immunotherapy for hepatocellular carcinoma using allogeneic suicide gene-modified killer cells.Mol Ther. 2014 Mar;22(3):634-644. doi: 10.1038/mt.2013.277. Epub 2013 Dec 6. Mol Ther. 2014. PMID: 24445938 Free PMC article.
-
A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer.Cancer Immunol Immunother. 2010 Dec;59(12):1781-9. doi: 10.1007/s00262-010-0904-3. Epub 2010 Aug 12. Cancer Immunol Immunother. 2010. PMID: 20703455 Free PMC article. Clinical Trial.
-
Allogeneic Natural Killer and Cytomegalovirus (CMV)-pp65 Pulsed Dendritic Cells Induced Complete Response Through 15 Months in a Patient with Recurrent Glioblastoma: A Case Study.Am J Case Rep. 2021 Mar 31;22:e931030. doi: 10.12659/AJCR.931030. Am J Case Rep. 2021. Retraction in: Am J Case Rep. 2022 Jul 05;23:e937680. doi: 10.12659/AJCR.937680. PMID: 33788825 Free PMC article. Retracted.
-
Complete remission of cancer in late-stage disease by radiation and transfer of allogeneic MHC-matched immune T cells: lessons from GvL studies in animals.Cancer Immunol Immunother. 2014 Jun;63(6):535-43. doi: 10.1007/s00262-014-1530-2. Epub 2014 Mar 9. Cancer Immunol Immunother. 2014. PMID: 24610041 Free PMC article. Review.
-
Abagovomab: an anti-idiotypic CA-125 targeted immunotherapeutic agent for ovarian cancer.Immunotherapy. 2011 Feb;3(2):153-62. doi: 10.2217/imt.10.100. Immunotherapy. 2011. PMID: 21322756 Free PMC article. Review.
References
-
- Weiden PL, Flournoy N, Sanders JE, Sullivan KM, Thomas ED. Antileukemic effect of graft-versus-host disease contributes to improved survival after allogeneic marrow transplantation. Transplant Proc. 1981;13:248–251. - PubMed
-
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. - PubMed
-
- Slavin S, Or R, Naparstek E, Ackerstein A, Weiss L. Cellular-mediated immunotherapy of leukemia in conjunction with autologous and allogeneic bone marrow transplantation in experimental animals and man. Blood. 1988;72(Suppl 1):407a.
-
- Slavin S, Or R, Kapelushnik Y, Drakos P, Ackerstein A, Vourka-Karussis U, et al. Immunotherapy of minimal residual disease in conjunction with autologous and allogeneic bone marrow transplantation (BMT) Leukemia. 1992;6(Suppl 4):164–166. - PubMed
-
- Slavin S, Naparstek E, Nagler A, Ackerstein A, Kapelushnik J, Or R. Allogeneic cell therapy for relapsed leukemia after bone marrow transplantation with donor peripheral blood lymphocytes. Exp Hematol. 1995;23:1553–1562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

