Impaired activation-induced cell death promotes spontaneous arthritis in antigen (cartilage proteoglycan)-specific T cell receptor-transgenic mice
- PMID: 20564001
- PMCID: PMC2952044
- DOI: 10.1002/art.27614
Impaired activation-induced cell death promotes spontaneous arthritis in antigen (cartilage proteoglycan)-specific T cell receptor-transgenic mice
Abstract
Objective: To investigate whether genetic preponderance of a T cell receptor (TCR) recognizing an arthritogenic peptide of human cartilage proteoglycan (PG) is sufficient for development of arthritis.
Methods: We performed a longitudinal study using BALB/c mice expressing a TCR that recognizes the arthritogenic ATEGRVRVNSAYQDK peptide of human cartilage PG. PG-specific TCR-transgenic (PG-TCR-Tg) mice were inspected weekly for peripheral arthritis until 12 months of age. Peripheral joints were examined histologically, and T cell responses, T cell activation markers, serum cytokines, and autoantibodies were measured. Apoptosis and signaling studies were performed in vitro on T cells from aged PG-TCR-Tg mice.
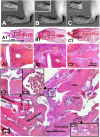
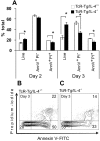
Results: Spontaneous arthritis developed as early as 5-6 months of age, and the incidence increased to 40-50% by 12 months of age. Progressive inflammation began with cartilage and bone erosions in the interphalangeal joints, and later expanded to the proximal joints of the front and hind paws. Spontaneous arthritis was associated with a high proportion of activated CD4+ T cells, enhanced interferon-γ and interleukin-17 (IL-17) production, and elevated levels of serum autoantibodies. PG-TCR-Tg mice lacking IL-4 developed arthritis earlier and at a higher incidence than IL-4-sufficient mice. Antigen-specific activation-induced cell death was diminished in vitro in CD4+ T cells of PG-TCR-Tg mice with spontaneous arthritis, especially in those lacking IL-4.
Conclusion: The presence of CD4+ T cells expressing a TCR specific for an arthritogenic PG epitope is sufficient to trigger spontaneous autoimmune inflammation in the joints of BALB/c mice. IL-4 appears to be a negative regulator of this disease, through attenuation of activation-induced cell death.
Figures


References
-
- Campbell IK, Kinkel SA, Drake SF, van Nieuwenhuijze A, Hubert FX, Tarlinton DM, et al. Autoimmune regulator controls T cell help for pathogenetic autoantibody production in collagen-induced arthritis. Arthritis Rheum. 2009;60:1683–93. - PubMed
-
- Trentham DE, Dynesius RA, Rocklin RE, David JR. Cellular sensitivity to collagen in rheumatoid arthritis. N Engl J Med. 1978;299:327–32. - PubMed
-
- Glant T, Csongor J, Szücs T. Immunopathologic role of proteoglycan antigens in rheumatoid joint diseases. Scand J Immunol. 1980;11:247–52. - PubMed
-
- Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunization against heterologous type II collagen induces arthritis in mice. Nature. 1980;282:666–8. - PubMed
-
- Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

