Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: the intergroup trial (RTOG 98-11)
- PMID: 20564111
- PMCID: PMC3831519
- DOI: 10.1002/cncr.25188
Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: the intergroup trial (RTOG 98-11)
Abstract
Background: Only 4 prospective randomized phase 3 trials have been reported for anal cancer. A prognostic factor analysis for anal cancer from a prospective database has been published from only 1 study (N = 110). To confirm and uncover new prognostic factors, we analyzed the prospective database of intergroup RTOG 98-11.
Methods: Univariate and multivariate analyses of the baseline characteristics for 5-year overall survival (OS) and disease-free survival (DFS) were carried out. Various combinations of tumor diameter and clinically positive nodes (N(+)) were analyzed to identify subgroups.
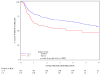
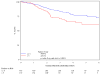
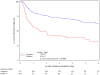
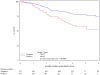
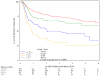
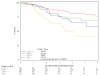
Results: A total of 644 were assessable and analyzed. Tumor diameter >5 cm was associated with poorer 5-year DFS (P = .0003) and poorer 5-year OS (P = .0031), and N(+) was associated with poorer 5-year DFS (P </= .0001) and poorer 5-year OS (P = </= .0001) in the multivariate analysis. In stratified analyses, N(+) had more adverse influence on DFS and OS than did tumor diameter. Patients with >5-cm tumor and N(+) had the worst DFS (only 30% at 3 years compared with 74% for the best group; <5 cm primary and N0) and OS (only 48% at 4 years compared with 81% for the best group; <5 cm primary and N0). Men had worse DFS (P = .02) and OS (P = .016). These factors maintained their influence in each treatment arm.
Conclusions: This prospective prognostic factor analysis establishes tumor diameter as an independent prognosticator of poorer 5-year DFS and OS and confirms N(+) and male sex as poor prognostic factors. This analysis also uncovers novel subgroups (derived from combining prognostic factors) with incremental worsening of DFS and OS.
Trial registration: ClinicalTrials.gov NCT00003596.
Cancer 2010. (c) 2010 American Cancer Society.
Figures
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Nigro ND, Seydel HG, Considine B, Vaitkevicius VK, Leichman L, Kinzie JJ. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51(10):1826–9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, 3rd, Thomas CR, Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. Jama. 2008;299(16):1914–21. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348(9034):1049–54. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15(5):2040–9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

