A phase 1 and pharmacodynamic study of decitabine in combination with carboplatin in patients with recurrent, platinum-resistant, epithelial ovarian cancer
- PMID: 20564122
- PMCID: PMC2930033
- DOI: 10.1002/cncr.25204
A phase 1 and pharmacodynamic study of decitabine in combination with carboplatin in patients with recurrent, platinum-resistant, epithelial ovarian cancer
Abstract
Background: Aberrant DNA methylation is a hallmark of cancer, and DNA methyltransferase inhibitors have demonstrated clinical efficacy in hematologic malignancies. On the basis of preclinical studies indicating that hypomethylating agents can reverse platinum resistance in ovarian cancer cells, the authors conducted a phase 1 trial of low-dose decitabine combined with carboplatin in patients with recurrent, platinum-resistant ovarian cancer.
Methods: Decitabine was administered intravenously daily for 5 days, before carboplatin (area under the curve, 5) on Day 8 of a 28-day cycle. By using a standard 3 + 3 dose escalation, decitabine was tested at 2 dose levels: 10 mg/m(2) (7 patients) or 20 mg/m(2) (3 patients). Peripheral blood mononuclear cells (PBMCs) and plasma collected on Days 1 (pretreatment), 5, 8, and 15 were used to assess global (LINE-1 repetitive element) and gene-specific DNA methylation.
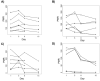
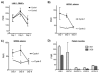
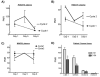
Results: Dose-limiting toxicity (DLT) at the 20-mg/m(2) dose was grade 4 neutropenia (2 patients), and no DLTs were observed at 10 mg/m(2). The most common toxicities were nausea, allergic reactions, neutropenia, fatigue, anorexia, vomiting, and abdominal pain, the majority being grades 1-2. One complete response was observed, and 3 additional patients had stable disease for >/=6 months. LINE-1 hypomethylation on Days 8 and 15 was detected in DNA from PBMCs. Of 5 ovarian cancer-associated methylated genes, HOXA11 and BRCA1 were demethylated in plasma on Days 8 and 15.
Conclusions: Repetitive low-dose decitabine is tolerated when combined with carboplatin in ovarian cancer patients, and demonstrates biological (ie, DNA-hypomethylating) activity, justifying further testing for clinical efficacy. Cancer 2010. (c) 2010 American Cancer Society.
Figures


References
-
- Bukowski RM, Ozols RF, Markman M. The management of recurrent ovarian cancer. Semin Oncol. 2007;34(2 Suppl 2):S1–15. - PubMed
-
- Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9(3):167–81. - PubMed
-
- Liu CM. Cancer of the ovary. N Engl J Med. 2005;352(12):1268–9. author reply 68-9. - PubMed
-
- Wei SH, Chen CM, Strathdee G, Harnsomburana J, Shyu CR, Rahmatpanah F, et al. Methylation microarray analysis of late-stage ovarian carcinomas distinguishes progression-free survival in patients and identifies candidate epigenetic markers. Clin Cancer Res. 2002;8(7):2246–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

