Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system
- PMID: 20564181
- PMCID: PMC2923828
- DOI: 10.1002/jcb.22592
Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system
Abstract
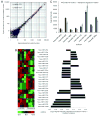
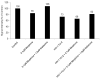
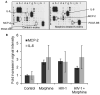
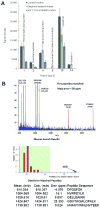
HIV-1-infected opiate abusers often exhibit an accelerated form of HIV-1-associated dementia and enhanced neurological dysfunction. Productive HIV-1 infection of microglia and perivascular macrophages and the resultant secretion of neurotoxic molecules by these cells contribute to this phenomenon. In order to understand the role of morphine in this process, we performed a genome-wide association study at the micro RNA (miRNA) and protein levels in human monocyte-derived macrophages (h-mdms). A total of 26 differentially expressed miRNA were identified (P < 0.01), of which hsa-miR-15b and hsa-miR-181b had the greatest increase and decrease in expression levels, respectively. Computational analysis predicted fibroblast growth factor-2 (FGF-2) as the strongest target gene for hsa-miR15b. Of note, we observed a decrease in FGF-2 protein expression in response to morphine. Both hsa-miR-15b and hsa-miR-181b have several predicted gene targets involved in inflammation and T-cell activation pathways. In this context, we observed induction of MCP-2 and IL-6 by morphine. Moreover, proteomic analysis revealed the induction of mitochondrial superoxide dismutase in response to morphine treatment. HIV-1 infection did not induce mitochondrial superoxide dismutase. Collectively, these observations demonstrate that morphine induces inflammation and oxidative stress in h-mdms thereby contributing to expansion of HIV-1 CNS reservoir expansion and disease progression. Of note, differentially expressed miRNAs (hsa-miR-15b and 181-b) may have a potential role in regulating these processes.
J. Cell. Biochem. 110: 834-845, 2010. (c) 2010 Wiley-Liss, Inc.
Figures

Similar articles
-
Morphine affects HIV-induced inflammatory response without influencing viral replication in human monocyte-derived macrophages.FEMS Immunol Med Microbiol. 2012 Mar;64(2):228-36. doi: 10.1111/j.1574-695X.2011.00894.x. FEMS Immunol Med Microbiol. 2012. PMID: 22066570 Free PMC article.
-
HIV-1 and opiates modulate miRNA profiles in extracellular vesicles.Front Immunol. 2023 Nov 9;14:1259998. doi: 10.3389/fimmu.2023.1259998. eCollection 2023. Front Immunol. 2023. PMID: 38022533 Free PMC article.
-
Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways.PLoS One. 2017 Mar 15;12(3):e0173292. doi: 10.1371/journal.pone.0173292. eCollection 2017. PLoS One. 2017. PMID: 28296904 Free PMC article.
-
MiRNA expression profiling in HIV pathogenesis, disease progression and response to treatment: a systematic review.Epigenomics. 2021 Oct;13(20):1653-1671. doi: 10.2217/epi-2021-0237. Epub 2021 Oct 25. Epigenomics. 2021. PMID: 34693727
-
Do opioids activate latent HIV-1 by down-regulating anti-HIV microRNAs?J Neuroimmune Pharmacol. 2012 Sep;7(3):519-23. doi: 10.1007/s11481-012-9356-1. Epub 2012 Apr 17. J Neuroimmune Pharmacol. 2012. PMID: 22527633 Review.
Cited by
-
The Effects of Opioids on HIV Neuropathogenesis.Front Immunol. 2019 Oct 18;10:2445. doi: 10.3389/fimmu.2019.02445. eCollection 2019. Front Immunol. 2019. PMID: 31681322 Free PMC article. Review.
-
MicroRNA-181b stimulates inflammation via the nuclear factor-κB signaling pathway in vitro.Exp Ther Med. 2015 Oct;10(4):1584-1590. doi: 10.3892/etm.2015.2702. Epub 2015 Aug 24. Exp Ther Med. 2015. PMID: 26622531 Free PMC article.
-
Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia.Pharmacol Rev. 2011 Sep;63(3):772-810. doi: 10.1124/pr.110.004135. Epub 2011 Jul 13. Pharmacol Rev. 2011. PMID: 21752874 Free PMC article. Review.
-
Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction.Cell Death Dis. 2012 Aug 30;3(8):e381. doi: 10.1038/cddis.2012.114. Cell Death Dis. 2012. PMID: 22932723 Free PMC article.
-
Interaction of drugs of abuse and microRNA with HIV: a brief review.Front Microbiol. 2015 Sep 29;6:967. doi: 10.3389/fmicb.2015.00967. eCollection 2015. Front Microbiol. 2015. PMID: 26483757 Free PMC article. Review.
References
-
- Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Bales RA, Ethisham A, Greenberg RN, Berger JR. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol. 2004;10:223–32. - PubMed
-
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–91. - PubMed
-
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Cytokine reversal of morphine-induced suppression of the antibody response. J Pharmacol Exp Ther. 1993;264:591–7. - PubMed
-
- Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol. 2009;40:101–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

