Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats
- PMID: 20564341
- PMCID: PMC2892197
- DOI: 10.1002/mc.20643
Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats
Abstract
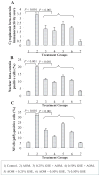
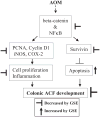
Chemoprevention by dietary agents/supplements has emerged as a novel approach to control various malignancies, including colorectal cancer (CRC). This study assessed dietary grape seed extract (GSE) effectiveness in preventing azoxymethane (AOM)-induced aberrant crypt foci (ACF) formation and associated mechanisms in Fischer 344 rats. Six-week-old rats were injected with AOM, and fed control diet or the one supplemented with 0.25% or 0.5% (w/w) GSE in pre- and post-AOM or only post-AOM experimental protocols. At 16 wk of age, rats were sacrificed and colons were evaluated for ACF formation followed by cell proliferation, apoptosis, and molecular analyses by immunohistochemistry. GSE-feeding caused strong chemopreventive efficacy against AOM-induced ACF formation in terms of up to 60% (P < 0.001) reduction in number of ACF and 66% (P < 0.001) reduction in crypt multiplicity. Mechanistic studies showed that GSE-feeding inhibited AOM-induced cell proliferation but enhanced apoptosis in colon including ACF, together with a strong decrease in cyclin D1, COX-2, iNOS, and survivin levels. Additional studies showed that GSE-feeding also decreased AOM-caused increase in beta-catenin and NF-kappaB levels in colon tissues. Compared to control animals, GSE alone treatment did not show any considerable change in these biological and molecular events in colon, and was nontoxic. Together, these findings show the chemopreventive efficacy of GSE against the early steps of colon carcinogenesis in rats via likely targeting of beta-catenin and NF-kappaB signaling, and suggest its potential usefulness for the prevention of human CRC.
Figures



References
-
- Huerta S. Recent advances in the molecular diagnosis and prognosis of colorectal cancer. Expert Rev Mol Diagn. 2008;8:277–288. - PubMed
-
- American Cancer Society. Colorectal cancer facts and figures. American Cancer Society; Atlanta, GA: 2009.
-
- Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–219. - PubMed
-
- Veeriah S, Miene C, Habermann N, et al. Apple polyphenols modulate expression of selected genes related to toxicological defense and stress response in human colon adenoma cells. Int J Cancer. 2008;122:2647–2655. - PubMed
-
- Rudolf E, Andelova H, Cervinka M. Polyphenolic compounds in chemoprevention of colon cancer - targets and signaling pathways. Anticancer Agents Med Chem. 2007;7:559–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

