Outcome of sustained virological responders with histologically advanced chronic hepatitis C
- PMID: 20564351
- PMCID: PMC2932862
- DOI: 10.1002/hep.23744
Outcome of sustained virological responders with histologically advanced chronic hepatitis C
Abstract
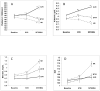
Retrospective studies suggest that subjects with chronic hepatitis C and advanced fibrosis who achieve a sustained virological response (SVR) have a lower risk of hepatic decompensation and hepatocellular carcinoma (HCC). In this prospective analysis, we compared the rate of death from any cause or liver transplantation, and of liver-related morbidity and mortality, after antiviral therapy among patients who achieved SVR, virologic nonresponders (NR), and those with initial viral clearance but subsequent breakthrough or relapse (BT/R) in the HALT-C (Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis) Trial. Laboratory and/or clinical outcome data were available for 140 of the 180 patients who achieved SVR. Patients with nonresponse (NR; n = 309) or who experienced breakthrough or relapse (BT/R; n = 77) were evaluated every 3 months for 3.5 years and then every 6 months thereafter. Outcomes included death, liver-related death, liver transplantation, decompensated liver disease, and HCC. Median follow-up for the SVR, BT/R, and NR groups of patients was 86, 85, and 79 months, respectively. At 7.5 years, the adjusted cumulative rate of death/liver transplantation and of liver-related morbidity/mortality in the SVR group (2.2% and 2.7%, respectively) was significantly lower than that of the NR group (21.3% and 27.2%, P < 0.001 for both) but not the BT/R group (4.4% and 8.7%). The adjusted hazard ratio (HR) for time to death/liver transplantation (HR = 0.17, 95% confidence interval [CI] = 0.06-0.46) or development of liver-related morbidity/mortality (HR = 0.15, 95% CI = 0.06-0.38) or HCC (HR = 0.19, 95% CI = 0.04-0.80) was significant for SVR compared to NR. Laboratory tests related to liver disease severity improved following SVR.
Conclusion: Patients with advanced chronic hepatitis C who achieved SVR had a marked reduction in death/liver transplantation, and in liver-related morbidity/mortality, although they remain at risk for HCC.
Trial registration: ClinicalTrials.gov NCT00006164.
Figures


References
-
- Camma C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–42. - PubMed
-
- Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–13. - PubMed
-
- Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87. - PubMed
-
- Imazeki F, Yokosuka O, Fukai K, Saisho H. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology. 2003;38:493–502. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01-DK-9-2325/DK/NIDDK NIH HHS/United States
- 1 UL1 RR024986/RR/NCRR NIH HHS/United States
- 1 UL1 RR025758-01/RR/NCRR NIH HHS/United States
- N01-DK-9-2321/DK/NIDDK NIH HHS/United States
- 1 UL1 RR024982-01/RR/NCRR NIH HHS/United States
- N01-DK-9-2320/DK/NIDDK NIH HHS/United States
- M01 RR006192/RR/NCRR NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- M01 RR000633/RR/NCRR NIH HHS/United States
- M01 RR000065/RR/NCRR NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- N01-DK-9-2322/DK/NIDDK NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- N01-DK-9-2323/DK/NIDDK NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- M01RR-00042/RR/NCRR NIH HHS/United States
- 1 UL1 RR 025780-01/RR/NCRR NIH HHS/United States
- M01RR-00065/RR/NCRR NIH HHS/United States
- N01-DK-9-2328/DK/NIDDK NIH HHS/United States
- N01-DK-9-2318/DK/NIDDK NIH HHS/United States
- N01-DK-9-2324/DK/NIDDK NIH HHS/United States
- N01-DK-9-2319/DK/NIDDK NIH HHS/United States
- M01RR-00051/RR/NCRR NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- M01RR-00827/RR/NCRR NIH HHS/United States
- N01-DK-9-2327/DK/NIDDK NIH HHS/United States
- M01RR-01066/RR/NCRR NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- N01-DK-9-2326/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- M01RR-00633/RR/NCRR NIH HHS/United States
- M01RR-00043/RR/NCRR NIH HHS/United States
- M01RR-06192/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
