Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures
- PMID: 20564721
- PMCID: PMC3763501
- DOI: 10.1002/lary.20930
Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures
Abstract
Objectives/hypothesis: The cystic fibrosis transmembrane conductance regulator (CFTR) serves as a predominant Cl(-) transport conduit in airway epithelium and is inhibited by cigarette smoke in vitro and in vivo. Activation of secondary Cl(-) transport pathways through calcium-activated Cl(-) channels (CaCC) has been postulated as a mechanism to bypass defects in CFTR-mediated transport. Because it is not known whether CaCCs are also inhibited by tobacco exposure, the current study was designed to investigate the effect of cigarette smoke condensate (CSC) on CaCC transport.
Study design: In vitro study.
Methods: Well-characterized primary murine nasal septal epithelial (MNSE) and human sinonasal epithelial (HSNE) cultures were exposed to CSC in Ussing chambers. We monitored CaCC short-circuit current through stimulation of P2Y purinergic receptors with uridine triphosphate or adenosine triphosphate and selective inhibition of the CFTR-dependent secretory pathway. Characterization of CaCC current was also accomplished in primary airway cells derived from transgenic CFTR(-/-) (knockout) murine models.
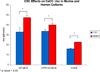
Results: Change in CaCC-mediated current (DeltaI(SC) representing transepithelial Ca-mediated Cl(-) secretion in muA/cm(2)) was significantly decreased in CSC-exposed wild type MNSE when compared to controls (32.8 +/- 4.6 vs. 47.5 +/- 2.3; respectively; P < .02). A similar effect was demonstrated in CFTR(-/-) MNSE cultures (33.4 +/- 2.8 vs. 38.6 +/- 2.0; P < .05>. HSNE cultures also had a significant reduction in I(SC) (16.1 +/- 0.6 vs. 22.7 +/- 0; P = .008).
Conclusions: CSC affects multiple pathways fundamental to airway ion transport, including both cyclic adenosine monophosphate and calcium activated Cl(-) transport. Inhibition of Cl(-) transport may contribute to common diseases of the airways, such as chronic rhinosinusitis and chronic obstructive pulmonary disease.
Conflict of interest statement
Conflict of Interest: Dr. Sorscher serves as a consultant for a Birmingham start up company (PNP Therapeutics, Inc) but is not employed by the company and draws no salary from the company. His consulting includes a role as Director/Officer. He acts as a consultant with the approval of UAB and the UAB CIRB. He is compensated for consulting with stock. This company has no relationship to the current manuscript. Dr. Sorscher and Dr. Woodworth are inventors on a patent submitted regarding the possible activity of chloride secretagogues for therapy of sinus disease (Provisional Patent Application Under 35 U.S.C. §111(b) and 37 C.F.R. § 1.53(c) in the United States Patent and Trademark Office). Dr. Woodworth is a consultant for Gyrus ENT, ArthroCare ENT, and is on the GlaxcoSmithKline speaker’s bureau.
Figures


Similar articles
-
Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency.Laryngoscope. 2015 Oct;125 Suppl 7(0 7):S1-S13. doi: 10.1002/lary.25335. Epub 2015 May 6. Laryngoscope. 2015. PMID: 25946147 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein.Laryngoscope. 2012 Jun;122(6):1193-7. doi: 10.1002/lary.23278. Epub 2012 Apr 20. Laryngoscope. 2012. PMID: 22522920 Free PMC article.
-
Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance.PLoS One. 2014 Aug 12;9(8):e104090. doi: 10.1371/journal.pone.0104090. eCollection 2014. PLoS One. 2014. PMID: 25117505 Free PMC article.
-
Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics.Am J Respir Med. 2003;2(4):299-309. doi: 10.1007/BF03256658. Am J Respir Med. 2003. PMID: 14719996 Review.
-
Adenosine receptors, cystic fibrosis, and airway hydration.Handb Exp Pharmacol. 2009;(193):363-81. doi: 10.1007/978-3-540-89615-9_12. Handb Exp Pharmacol. 2009. PMID: 19639288 Review.
Cited by
-
Acquired cilia dysfunction in chronic rhinosinusitis.Am J Rhinol Allergy. 2012 Jan-Feb;26(1):1-6. doi: 10.2500/ajra.2012.26.3716. Am J Rhinol Allergy. 2012. PMID: 22391065 Free PMC article. Review.
-
Correlation of T2R38 taste phenotype and in vitro biofilm formation from nonpolypoid chronic rhinosinusitis patients.Int Forum Allergy Rhinol. 2016 Aug;6(8):783-91. doi: 10.1002/alr.21803. Epub 2016 Jun 16. Int Forum Allergy Rhinol. 2016. PMID: 27309535 Free PMC article.
-
Lipopolysaccharide Causes Acquired CFTR Dysfunction in Murine Nasal Airways.Otolaryngol Head Neck Surg. 2025 May;172(5):1774-1780. doi: 10.1002/ohn.1143. Epub 2025 Jan 26. Otolaryngol Head Neck Surg. 2025. PMID: 39865444 Free PMC article.
-
Differential Chloride Secretory Capacity in Transepithelial Ion Transport Properties in Chronic Rhinosinusitis.Am J Rhinol Allergy. 2020 Nov;34(6):830-837. doi: 10.1177/1945892420930975. Epub 2020 Jun 23. Am J Rhinol Allergy. 2020. PMID: 32576027 Free PMC article.
-
Risk Factors and Comorbidities in Chronic Rhinosinusitis.Curr Allergy Asthma Rep. 2016 Feb;16(2):16. doi: 10.1007/s11882-015-0589-y. Curr Allergy Asthma Rep. 2016. PMID: 26800681 Review.
References
-
- Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effect on the physical properties of airway mucus. Am J Physiol. 1998;274:L258–L263. - PubMed
-
- Anderson MP, Gregory RJ, Thompson S, et al. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. - PubMed
-
- Agius AM, Smallman LA, Pahor AL. Age, smoking and nasal ciliary beat frequency. Clin Otolaryngol. 1998;23:227–230. - PubMed
-
- Dalhamn T, Rylander R. Ciliotoxicity of cigar and cigarette smoke. Arch Environ Health. 1970;20:252–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

