Biochemical and structural characterization of a ureidoglycine aminotransferase in the Klebsiella pneumoniae uric acid catabolic pathway
- PMID: 20565126
- PMCID: PMC2912414
- DOI: 10.1021/bi1006755
Biochemical and structural characterization of a ureidoglycine aminotransferase in the Klebsiella pneumoniae uric acid catabolic pathway
Abstract
Many plants, fungi, and bacteria catabolize allantoin as a mechanism for nitrogen assimilation. Recent reports have shown that in plants and some bacteria the product of hydrolysis of allantoin by allantoinase is the unstable intermediate ureidoglycine. While this molecule can spontaneously decay, genetic analysis of some bacterial genomes indicates that an aminotransferase may be present in the pathway. Here we present evidence that Klebsiella pneumoniae HpxJ is an aminotransferase that preferentially converts ureidoglycine and an alpha-keto acid into oxalurate and the corresponding amino acid. We determined the crystal structure of HpxJ, allowing us to present an explanation for substrate specificity.
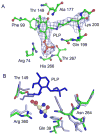
Figures


Similar articles
-
Biochemical and structural characterization of Klebsiella pneumoniae oxamate amidohydrolase in the uric acid degradation pathway.Acta Crystallogr D Struct Biol. 2016 Jun;72(Pt 6):808-16. doi: 10.1107/S2059798316007099. Epub 2016 May 25. Acta Crystallogr D Struct Biol. 2016. PMID: 27303801 Free PMC article.
-
Chemical basis of nitrogen recovery through the ureide pathway: formation and hydrolysis of S-ureidoglycine in plants and bacteria.ACS Chem Biol. 2010 Feb 19;5(2):203-14. doi: 10.1021/cb900248n. ACS Chem Biol. 2010. PMID: 20038185
-
Structural and mechanistic studies on Klebsiella pneumoniae 2-Oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline decarboxylase.J Biol Chem. 2010 Nov 12;285(46):35446-54. doi: 10.1074/jbc.M110.156034. Epub 2010 Sep 8. J Biol Chem. 2010. PMID: 20826786 Free PMC article.
-
Catabolism of caffeine in plants and microorganisms.Front Biosci. 2004 May 1;9:1348-59. doi: 10.2741/1339. Front Biosci. 2004. PMID: 14977550 Review.
-
Update on ureide degradation in legumes.J Exp Bot. 2006;57(1):5-12. doi: 10.1093/jxb/erj013. Epub 2005 Nov 29. J Exp Bot. 2006. PMID: 16317038 Review.
Cited by
-
The multi-drug efflux system AcrABZ-TolC is essential for infection of Salmonella Typhimurium by the flagellum-dependent bacteriophage Chi.J Virol. 2021 May 10;95(11):e00394-21. doi: 10.1128/JVI.00394-21. Epub 2021 Mar 17. J Virol. 2021. PMID: 33731456 Free PMC article.
-
An aminotransferase branch point connects purine catabolism to amino acid recycling.Nat Chem Biol. 2010 Nov;6(11):801-6. doi: 10.1038/nchembio.445. Epub 2010 Sep 19. Nat Chem Biol. 2010. PMID: 20852637
-
The purinosome, a multi-protein complex involved in the de novo biosynthesis of purines in humans.Chem Commun (Camb). 2013 May 18;49(40):4444-52. doi: 10.1039/c3cc41437j. Epub 2013 Apr 11. Chem Commun (Camb). 2013. PMID: 23575936 Free PMC article.
-
Structural and kinetic insights into the mechanism of 5-hydroxyisourate hydrolase from Klebsiella pneumoniae.Acta Crystallogr D Biol Crystallogr. 2011 Aug;67(Pt 8):671-7. doi: 10.1107/S090744491101746X. Epub 2011 Jul 12. Acta Crystallogr D Biol Crystallogr. 2011. PMID: 21795808 Free PMC article.
-
Alternative conformation induced by substrate binding for Arabidopsis thalianaN6-methyl-AMP deaminase.Nucleic Acids Res. 2019 Apr 8;47(6):3233-3243. doi: 10.1093/nar/gkz070. Nucleic Acids Res. 2019. PMID: 30721978 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

