Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling
- PMID: 20565132
- PMCID: PMC2957295
- DOI: 10.1021/bi100517x
Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling
Abstract
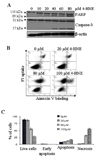
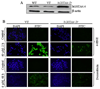
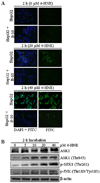
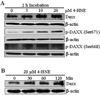
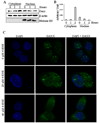
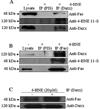
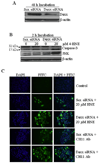
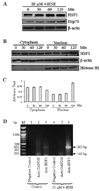
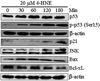
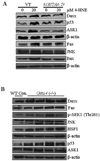
In recent years, 4-hydroxy-2-nonenal (4-HNE) has emerged as an important second messenger in cell cycle signaling. Here, we demonstrate that 4-HNE induces signaling for apoptosis via both the Fas-mediated extrinsic and the p53-mediated intrinsic pathways in HepG2 cells. 4-HNE induces a Fas-mediated DISC independent apoptosis pathway by activating ASK1, JNK, and caspase-3. Parallel treatment of 4-HNE to HepG2 cells also induces apoptosis by the p53 pathway through activation of Bax, p21, JNK, and caspase-3. Exposure of HepG2 cells to 4-HNE leads to the activation of both Fas and Daxx, promotes the export of Daxx from the nucleus to cytoplasm, and facilitates Fas-Daxx binding. Depletion of Daxx by siRNA results in the potentiation of apoptosis, indicating that Fas-Daxx binding in fact is inhibitory to Fas-mediated apoptosis in cells. 4-HNE-induced translocation of Daxx is also accompanied by the activation and nuclear accumulation of HSF1 and up-regulation of heat shock protein Hsp70. All these effects of 4-HNE in cells can be attenuated by ectopic expression of hGSTA4-4, the isozyme of glutathione S-transferase with high activity for 4-HNE. Through immunoprecipitation and liquid chromatography-tandem mass spectrometry, we have demonstrated the covalent binding of 4-HNE to Daxx. We also demonstrate that 4-HNE modification induces phosphorylation of Daxx at Ser668 and Ser671 to facilitate its cytoplasmic export. These results indicate that while 4-HNE exhibits toxicity through several mechanisms, in parallel it evokes signaling for defense mechanisms to self-regulate its toxicity and can simultaneously affect multiple signaling pathways through its interactions with membrane receptors and transcription factors/repressors.
Figures

Similar articles
-
4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to Fas.Biochemistry. 2008 Jan 8;47(1):143-56. doi: 10.1021/bi701559f. Epub 2007 Dec 11. Biochemistry. 2008. PMID: 18069800 Free PMC article.
-
A suppressive role of the prolyl isomerase Pin1 in cellular apoptosis mediated by the death-associated protein Daxx.J Biol Chem. 2007 Dec 14;282(50):36671-81. doi: 10.1074/jbc.M704145200. Epub 2007 Oct 15. J Biol Chem. 2007. PMID: 17938171
-
Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death.Free Radic Biol Med. 2008 Jul 15;45(2):111-8. doi: 10.1016/j.freeradbiomed.2008.04.007. Epub 2008 May 2. Free Radic Biol Med. 2008. PMID: 18456001 Free PMC article. Review.
-
Inhibition of Daxx-mediated apoptosis by heat shock protein 27.Mol Cell Biol. 2000 Oct;20(20):7602-12. doi: 10.1128/MCB.20.20.7602-7612.2000. Mol Cell Biol. 2000. PMID: 11003656 Free PMC article.
-
The Daxx enigma.Apoptosis. 2000 Jun;5(3):217-20. doi: 10.1023/a:1009696227420. Apoptosis. 2000. PMID: 11225842 Review.
Cited by
-
4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway.J Biol Chem. 2013 Jul 12;288(28):20532-46. doi: 10.1074/jbc.M113.467662. Epub 2013 Jun 3. J Biol Chem. 2013. PMID: 23733185 Free PMC article.
-
Iron homeostasis and post-hemorrhagic hydrocephalus: a review.Front Neurol. 2024 Jan 12;14:1287559. doi: 10.3389/fneur.2023.1287559. eCollection 2023. Front Neurol. 2024. PMID: 38283681 Free PMC article. Review.
-
Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling.Toxicol Appl Pharmacol. 2015 Dec 15;289(3):361-70. doi: 10.1016/j.taap.2015.10.006. Epub 2015 Oct 23. Toxicol Appl Pharmacol. 2015. PMID: 26476300 Free PMC article. Review.
-
RLIP76 Inhibition: A Promising Developmental Therapy for Neuroblastoma.Pharm Res. 2017 Aug;34(8):1673-1682. doi: 10.1007/s11095-017-2154-y. Epub 2017 Apr 6. Pharm Res. 2017. PMID: 28386633 Review.
-
Role of 4-hydroxynonenal in chemopreventive activities of sulforaphane.Free Radic Biol Med. 2012 Jun 1-15;52(11-12):2177-85. doi: 10.1016/j.freeradbiomed.2012.04.012. Epub 2012 Apr 23. Free Radic Biol Med. 2012. PMID: 22579574 Free PMC article. Review.
References
-
- Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J. Biol. Chem. 2001;276:41213–41223. - PubMed
-
- Yang Y, Sharma A, Sharma R, Patrick B, Singhal SS, Zimniak P, Awasthi S, Awasthi YC. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J. Biol. Chem. 2003;278:41380–41388. - PubMed
-
- Dianzani MU. 4-hydroxynonenal from pathology to physiology. Mol. Aspects Med. 2003;24:263–272. - PubMed
-
- Nakashima I, Liu W, Akhand AA, Takeda K, Kawamoto Y, Kato M, Suzuki H. 4-Hydroxynonenal triggers multistep signal transduction cascades for suppression of cellular functions. Mol. Aspects Med. 2003;24:231–238. - PubMed
-
- Barrera G, Pizzimenti S, Dianzani MU. 4-Hydroxynonenal and regulation of cell cycle: effects on the pRb/E2F pathway. Free Radic. Biol. Med. 2004;37:597–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

