Biliverdin amides reveal roles for propionate side chains in bilin reductase recognition and in holophytochrome assembly and photoconversion
- PMID: 20565135
- PMCID: PMC2925249
- DOI: 10.1021/bi100756x
Biliverdin amides reveal roles for propionate side chains in bilin reductase recognition and in holophytochrome assembly and photoconversion
Abstract
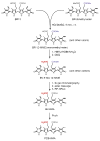
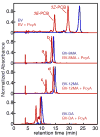
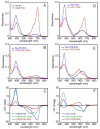
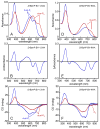
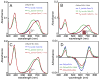
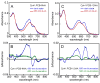
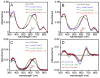
Linear tetrapyrroles (bilins) perform important antioxidant and light-harvesting functions in cells from bacteria to humans. To explore the role of the propionate moieties in bilin metabolism, we report the semisynthesis of mono- and diamides of biliverdin IXalpha and those of its non-natural XIIIalpha isomer. Initially, these were examined as substrates of two types of NADPH-dependent biliverdin reductase, BVR and BvdR, and of the representative ferredoxin-dependent bilin reductase, phycocyanobilin:ferredoxin oxidoreductase (PcyA). Our studies indicate that the NADPH-dependent biliverdin reductases are less accommodating to amidation of the propionic acid side chains of biliverdin IXalpha than PcyA, which does not require free carboxylic acid side chains to yield its phytobilin product, phycocyanobilin. Bilin amides were also assembled with BV-type and phytobilin-type apophytochromes, demonstrating a role for the 8-propionate in the formation of the spectroscopically native P(r) dark states of these biliprotein photosensors. Neither ionizable propionate side chain proved to be essential to primary photoisomerization for both classes of phytochromes, but an unsubstituted 12-propionate was required for full photointerconversion of phytobilin-type phytochrome Cph1. Taken together, these studies provide insight into the roles of the ionizable propionate side chains in substrate discrimination by two bilin reductase families while further underscoring the mechanistic differences between the photoconversions of BV-type and phytobilin-type phytochromes.
Figures
Similar articles
-
Biliverdin reduction by cyanobacterial phycocyanobilin:ferredoxin oxidoreductase (PcyA) proceeds via linear tetrapyrrole radical intermediates.J Am Chem Soc. 2004 Jul 21;126(28):8682-93. doi: 10.1021/ja049280z. J Am Chem Soc. 2004. PMID: 15250720
-
Structural insights into vinyl reduction regiospecificity of phycocyanobilin:ferredoxin oxidoreductase (PcyA).J Biol Chem. 2010 Jan 8;285(2):1000-7. doi: 10.1074/jbc.M109.055632. Epub 2009 Nov 2. J Biol Chem. 2010. PMID: 19887371 Free PMC article.
-
Crystal structure of phytochromobilin synthase in complex with biliverdin IXα, a key enzyme in the biosynthesis of phytochrome.J Biol Chem. 2020 Jan 17;295(3):771-782. doi: 10.1074/jbc.RA119.011431. Epub 2019 Dec 10. J Biol Chem. 2020. PMID: 31822504 Free PMC article.
-
Bilin-metabolizing enzymes: site-specific reductions catalyzed by two different type of enzymes.Curr Opin Struct Biol. 2019 Dec;59:73-80. doi: 10.1016/j.sbi.2019.03.005. Epub 2019 Apr 5. Curr Opin Struct Biol. 2019. PMID: 30954759 Review.
-
Function and distribution of bilin biosynthesis enzymes in photosynthetic organisms.Photochem Photobiol Sci. 2008 Oct;7(10):1121-30. doi: 10.1039/b807209b. Epub 2008 Jul 7. Photochem Photobiol Sci. 2008. PMID: 18846276 Review.
Cited by
-
Circular dichroism spectroscopy reveals multiple phytochrome photoproducts in equilibrium.Photochem Photobiol Sci. 2025 Jul 18. doi: 10.1007/s43630-025-00763-2. Online ahead of print. Photochem Photobiol Sci. 2025. PMID: 40681765
-
Green/red cyanobacteriochromes regulate complementary chromatic acclimation via a protochromic photocycle.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):4974-9. doi: 10.1073/pnas.1302909110. Epub 2013 Mar 11. Proc Natl Acad Sci U S A. 2013. PMID: 23479641 Free PMC article.
-
Conserved histidine and tyrosine determine spectral responses through the water network in Deinococcus radiodurans phytochrome.Photochem Photobiol Sci. 2022 Nov;21(11):1975-1989. doi: 10.1007/s43630-022-00272-6. Epub 2022 Jul 29. Photochem Photobiol Sci. 2022. PMID: 35906527
-
Spectral and photochemical diversity of tandem cysteine cyanobacterial phytochromes.J Biol Chem. 2020 May 8;295(19):6754-6766. doi: 10.1074/jbc.RA120.012950. Epub 2020 Mar 17. J Biol Chem. 2020. PMID: 32184354 Free PMC article.
-
Rational Construction of Compact de Novo-Designed Biliverdin-Binding Proteins.Biochemistry. 2018 Dec 11;57(49):6752-6756. doi: 10.1021/acs.biochem.8b01076. Epub 2018 Nov 28. Biochemistry. 2018. PMID: 30468389 Free PMC article.
References
-
- Frankenberg NF, Lagarias JC. Biosynthesis and biological function of bilins. In: Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook Chlorophylls and Bilins: Biosynthesis Structure and Degradation. Academic Press; New York: 2003. pp. 211–235.
-
- Foresti R, Green CJ, Motterlini R. Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem Soc Symp. 2004:177–192. - PubMed
-
- Wilks A. Heme oxygenase: evolution, structure, and mechanism. Antioxid Redox Signal. 2002;4:603–614. - PubMed
-
- McDonagh AF. Turning green to gold. Nature Struct Biol. 2001;8:198–200. - PubMed
-
- Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Tr Pharm Sci. 2009;30:129–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

