Biochemical markers identify influences on bone and cartilage degradation in osteoarthritis--the effect of sex, Kellgren-Lawrence (KL) score, body mass index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation
- PMID: 20565725
- PMCID: PMC2902412
- DOI: 10.1186/1471-2474-11-125
Biochemical markers identify influences on bone and cartilage degradation in osteoarthritis--the effect of sex, Kellgren-Lawrence (KL) score, body mass index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation
Abstract
Background: Osteoarthritis (OA) involves changes in both bone and cartilage. These processes might be associated under some circumstances. This study investigated correlations between bone and cartilage degradation in patients with OA as a function of sex, Kellgren-Lawrence (KL) score, Body Mass Index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation.
Methods: This study was a 2-week, double-blind, double-dummy, randomized study including 37 postmenopausal women and 36 men, aged 57-75 years, with painful knee OA, and a KL-score of I - III. Subjects were allocated to one of three treatment arms: 0.6 mg or 0.8 mg oral sCT, or placebo given twice-daily for 14 days. Correlations between gender, KL score, or BMI and the bone resorption marker, serum C-terminal telopeptide of collagen type I (CTX-I), or the cartilage degradation marker, urine C-terminal telopeptide of collagen type II (CTX-II) were investigated.
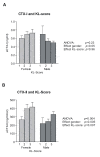
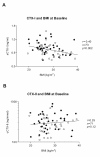
Results: At baseline, biomarkers indicated women with OA experienced higher bone and cartilage degradation than men. CTX-I levels were significantly higher, and CTX-II levels only marginally higher, in women than in men (p = 0.04 and p = 0.06, respectively). Increasing KL score was not correlated with bone resorption, but was significantly associated with the cartilage degradation CTX-II marker in both men and women (p = 0.007). BMI was significantly and negatively correlated to the bone resorption marker CTX-I, r = -0.40 (p = 0.002), but showed only a borderline positive correlation to CTX-II, r = 0.25 (p = 0.12). Before morning treatments on days 1 and 14, no correlation was seen between CTX-I and CTX-II in either the sCT or placebo group. However, oral sCT and food intake induced a clear correlation between these bone and cartilage degradation markers. Four hours after the first sCT dose on treatment days 1 and 14, a significant correlation (r = 0.71, p < 0.001) between changes in both CTX-I and CTX-II was seen. In the placebo group a weakly significant correlation between changes in both markers was found on day 1 (r = 0.49, p = 0.02), but not on day 14.
Conclusion: Bone resorption was higher in females than males, while cartilage degradation was correlated with increasing KL-score and only weakly associated with BMI. Bone and cartilage degradation were not correlated in untreated individuals, but dosing with oral sCT with or without food, and a mid-day meal, decreased bone and cartilage degradation and induced a correlation between both markers. Changes in bone and cartilage markers may aid in the identification of potential new treatment opportunities for OA.
Trial registration: Clinical trial registration number (EUDRACT2006-005532-24 & NCT00486369).
Figures



Similar articles
-
The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: a 14-day randomized study.Osteoarthritis Cartilage. 2010 Feb;18(2):150-9. doi: 10.1016/j.joca.2009.08.004. Epub 2009 Sep 1. Osteoarthritis Cartilage. 2010. PMID: 19747581 Clinical Trial.
-
Treatment of symptomatic knee osteoarthritis with oral salmon calcitonin: results from two phase 3 trials.Osteoarthritis Cartilage. 2015 Apr;23(4):532-43. doi: 10.1016/j.joca.2014.12.019. Epub 2015 Jan 9. Osteoarthritis Cartilage. 2015. PMID: 25582279 Clinical Trial.
-
Associations between biomarkers of bone and cartilage turnover, gender, pain categories and radiographic severity in knee osteoarthritis.Arthritis Res Ther. 2019 Sep 3;21(1):203. doi: 10.1186/s13075-019-1987-7. Arthritis Res Ther. 2019. PMID: 31481084 Free PMC article. Clinical Trial.
-
C-Terminal Cross-Linked Telopeptides of Type II Collagen as Biomarker for Radiological Knee Osteoarthritis: A Meta-Analysis.Cartilage. 2020 Oct;11(4):512-520. doi: 10.1177/1947603518798884. Epub 2018 Sep 15. Cartilage. 2020. PMID: 30221987 Free PMC article. Review.
-
Effects of risedronate on osteoarthritis of the knee.Yonsei Med J. 2010 Mar;51(2):164-70. doi: 10.3349/ymj.2010.51.2.164. Epub 2010 Feb 12. Yonsei Med J. 2010. PMID: 20191005 Free PMC article. Review.
Cited by
-
The relationship between urinary C-Telopeptide fragments of type II collagen, knee joint load, pain, and physical function in individuals with medial knee osteoarthritis.Braz J Phys Ther. 2021 Jan-Feb;25(1):62-69. doi: 10.1016/j.bjpt.2020.02.002. Epub 2020 Feb 26. Braz J Phys Ther. 2021. PMID: 32151525 Free PMC article.
-
Personalized medicine for osteoarthritis: where are we now?Ther Adv Musculoskelet Dis. 2013 Apr;5(2):67-75. doi: 10.1177/1759720X12470752. Ther Adv Musculoskelet Dis. 2013. PMID: 23641258 Free PMC article.
-
Calcitonin treatment for osteoarthritis and rheumatoid arthritis - a systematic review and meta-analysis of preclinical data.EFORT Open Rev. 2024 Jul 1;9(7):600-614. doi: 10.1530/EOR-23-0133. EFORT Open Rev. 2024. PMID: 38949173 Free PMC article.
-
Serum Levels of Coll2-1, a Specific Biomarker of Cartilage Degradation, Are Not Affected by Sampling Conditions, Circadian Rhythm, and Seasonality.Cartilage. 2021 Dec;13(1_suppl):540S-549S. doi: 10.1177/1947603519878489. Epub 2019 Oct 20. Cartilage. 2021. PMID: 31631693 Free PMC article.
-
Which Predicts Quadriceps Muscle Strength in Knee Osteoarthritis: Biological Markers or Clinical Variables?Arch Rheumatol. 2016 Dec 15;32(1):32-38. doi: 10.5606/ArchRheumatol.2017.5919. eCollection 2017 Mar. Arch Rheumatol. 2016. PMID: 30375528 Free PMC article.
References
-
- Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–1206. doi: 10.1002/art.20124. - DOI - PubMed
-
- Mouritzen U, Christgau S, Lehmann HJ, Tanko LB, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62:332–336. doi: 10.1136/ard.62.4.332. - DOI - PMC - PubMed
-
- Calvo E, Castaneda S, Largo R, Fernandez-Valle ME, Rodriguez-Salvanes F, Herrero-Beaumont G. Osteoporosis increases the severity of cartilage damage in an experimental model of osteoarthritis in rabbits. Osteoarthritis Cartilage. 2006. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

