Proteomic analysis of sea urchin (Strongylocentrotus purpuratus) spicule matrix
- PMID: 20565753
- PMCID: PMC2909932
- DOI: 10.1186/1477-5956-8-33
Proteomic analysis of sea urchin (Strongylocentrotus purpuratus) spicule matrix
Abstract
Background: The sea urchin embryo has been an important model organism in developmental biology for more than a century. This is due to its relatively simple construction, translucent appearance, and the possibility to follow the fate of individual cells as development to the pluteus larva proceeds. Because the larvae contain tiny calcitic skeletal elements, the spicules, they are also important model organisms for biomineralization research. Similar to other biominerals the spicule contains an organic matrix, which is thought to play an important role in its formation. However, only few spicule matrix proteins were identified previously.
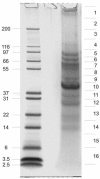
Results: Using mass spectrometry-based methods we have identified 231 proteins in the matrix of the S. purpuratus spicule matrix. Approximately two thirds of the identified proteins are either known or predicted to be extracellular proteins or transmembrane proteins with large ectodomains. The ectodomains may have been solubilized by partial proteolysis and subsequently integrated into the growing spicule. The most abundant protein of the spicule matrix is SM50. SM50-related proteins, SM30-related proteins, MSP130 and related proteins, matrix metalloproteases and carbonic anhydrase are among the most abundant components.
Conclusions: The spicule matrix is a relatively complex mixture of proteins not only containing matrix-specific proteins with a function in matrix assembly or mineralization, but also: 1) proteins possibly important for the formation of the continuous membrane delineating the mineralization space; 2) proteins for secretory processes delivering proteinaceous or non-proteinaceous precursors; 3) or proteins reflecting signaling events at the cell/matrix interface. Comparison of the proteomes of different skeletal matrices allows prediction of proteins of general importance for mineralization in sea urchins, such as SM50, SM30-E, SM29 or MSP130. The comparisons also help point out putative tissue-specific proteins, such as tooth phosphodontin or specific spicule matrix metalloproteases of the MMP18/19 group. Furthermore, the direct sequence analysis of peptides by MS/MS validates many predicted genes and confirms the existence of the corresponding proteins.
Figures

Similar articles
-
The sea urchin (Strongylocentrotus purpuratus) test and spine proteomes.Proteome Sci. 2008 Aug 11;6:22. doi: 10.1186/1477-5956-6-22. Proteome Sci. 2008. PMID: 18694502 Free PMC article.
-
Expression of spicule matrix proteins in the sea urchin embryo during normal and experimentally altered spiculogenesis.Dev Biol. 2000 Sep 1;225(1):201-13. doi: 10.1006/dbio.2000.9828. Dev Biol. 2000. PMID: 10964475
-
Spiculogenesis in the sea urchin embryo: Studies on the SM30 spicule matrix protein.Dev Growth Differ. 1995 Feb;37(1):69-78. doi: 10.1046/j.1440-169X.1995.00008.x. Dev Growth Differ. 1995. PMID: 37281595
-
Biomineralization of the spicules of sea urchin embryos.Zoolog Sci. 2002 Mar;19(3):253-61. doi: 10.2108/zsj.19.253. Zoolog Sci. 2002. PMID: 12125922 Review.
-
Matrix and mineral in the sea urchin larval skeleton.J Struct Biol. 1999 Jun 30;126(3):216-26. doi: 10.1006/jsbi.1999.4105. J Struct Biol. 1999. PMID: 10475684 Review.
Cited by
-
In-depth proteomic analysis of a mollusc shell: acid-soluble and acid-insoluble matrix of the limpet Lottia gigantea.Proteome Sci. 2012 Jun 13;10(1):28. doi: 10.1186/1477-5956-10-28. Proteome Sci. 2012. PMID: 22540284 Free PMC article.
-
Sea Urchin Spicule Matrix Proteins Form Mesoscale "Smart" Hydrogels That Exhibit Selective Ion Interactions.ACS Omega. 2017 Sep 26;2(9):6151-6158. doi: 10.1021/acsomega.7b00719. eCollection 2017 Sep 30. ACS Omega. 2017. PMID: 31457861 Free PMC article.
-
Evolution of Epidermal Growth Factor (EGF)-like and Zona Pellucida Domains Containing Shell Matrix Proteins in Mollusks.Mol Biol Evol. 2022 Jul 2;39(7):msac148. doi: 10.1093/molbev/msac148. Mol Biol Evol. 2022. PMID: 35796746 Free PMC article.
-
Deep conservation of bivalve nacre proteins highlighted by shell matrix proteomics of the Unionoida Elliptio complanata and Villosa lienosa.J R Soc Interface. 2017 Jan;14(126):20160846. doi: 10.1098/rsif.2016.0846. J R Soc Interface. 2017. PMID: 28123096 Free PMC article.
-
Mineral Composition of Skeletal Elements in Dorid Nudibranchia Onchidoris muricata (Gastropoda, Mollusca).Biomimetics (Basel). 2025 Mar 29;10(4):211. doi: 10.3390/biomimetics10040211. Biomimetics (Basel). 2025. PMID: 40277610 Free PMC article.
References
-
- Okazaki K. Skeleton formation of sea urchin larvae II. Organic matrix of the spicule. Embryologia. 1960;5:283–320. doi: 10.1111/j.1440-169X.1960.tb00096.x. - DOI
-
- Decker GL, Lennarz WJ. Skeletogenesis in the sea urchin embryo. Development. 1988;103:231–247. - PubMed
-
- Wilt FH, Ettensohn CA. In: Handbook of Biomineralization. Bäuerlein E, editor. Vol. 1. Weinheim:Wiley-VCH Verlag; 2007. The morphogenesis and biomineralization of the sea urchin larval skeleton; pp. 183–210.
LinkOut - more resources
Full Text Sources
Miscellaneous

