A novel role of the aryl hydrocarbon receptor (AhR) in centrosome amplification - implications for chemoprevention
- PMID: 20565777
- PMCID: PMC2898706
- DOI: 10.1186/1476-4598-9-153
A novel role of the aryl hydrocarbon receptor (AhR) in centrosome amplification - implications for chemoprevention
Abstract
Background: Centrosome aberrations can cause genomic instability and correlate with malignant progression in common human malignancies such as breast and prostate cancer. Deregulation of cyclin/cyclin-dependent kinase 2 (CDK2) activity has previously been shown to be critically involved in centrosome overduplication. We therefore test here whether small molecule CDK inhibitors derived from the bis-indole indirubin can be used to suppress centrosome aberrations as a novel approach to chemoprevention of malignant progression.
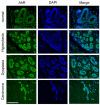
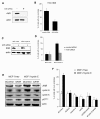
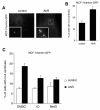
Results: As expected, we found that the CDK inhibitor indirubin-3'-oxime (IO) suppresses centrosome amplification in breast cancer cells. However, we made the unexpected discovery that indirubin-derived compounds that have been chemically modified to be inactive as kinase inhibitors such as 1-methyl-indirubin-3'-oxime (MeIO) still significantly reduced centrosome amplification. All indirubins used in the present study are potent agonists of the aryl hydrocarbon receptor (AhR), which is known for its important role in the cellular metabolism of xenobiotics. To corroborate our results, we first show that the coincidence of nuclear AhR overexpression, reflecting a constitutive activation, and numerical centrosome aberrations correlates significantly with malignancy in mammary tissue specimens. Remarkably, a considerable proportion (72.7%) of benign mammary tissue samples scored also positive for nuclear AhR overexpression. We furthermore provide evidence that continued expression of endogenous AhR is critical to promote centriole overduplication induced by cyclin E and that AhR and cyclin E may function in the same pathway. Overexpression of the AhR in the absence of exogenous ligands was found to rapidly disrupt centriole duplication control. Nonetheless, the AhR agonists IO and MeIO were still found to significantly reduce centriole overduplication stimulated by ectopic AhR expression.
Conclusions: Our results indicate that continued expression of endogenous AhR promotes centrosome amplification in breast cancer cells in a pathway that involves cyclin E. AhR agonists such as indirubins inhibit centrosome amplification even when stimulated by ectopic expression of the AhR suggesting that these compounds are potentially useful for the chemoprevention of centrosome-mediated cell division errors and malignant progression in neoplasms in which the AhR is overexpressed. Future studies are warranted to determine whether individuals in which nuclear AhR overexpression is detected in benign mammary tissue are at a higher risk for developing pre-cancerous or cancerous breast lesions.
Figures


Similar articles
-
Independent actions on cyclin-dependent kinases and aryl hydrocarbon receptor mediate the antiproliferative effects of indirubins.Oncogene. 2004 May 27;23(25):4400-12. doi: 10.1038/sj.onc.1207535. Oncogene. 2004. PMID: 15077192
-
Cyclin-dependent kinase inhibitor indirubin-3'-oxime selectively inhibits human papillomavirus type 16 E7-induced numerical centrosome anomalies.Oncogene. 2004 Oct 28;23(50):8206-15. doi: 10.1038/sj.onc.1208012. Oncogene. 2004. PMID: 15378001
-
Activation of the aryl hydrocarbon receptor by 3-methylcholanthrene, but not by indirubin, suppresses mammosphere formation via downregulation of CDC20 expression in breast cancer cells.Biochem Biophys Res Commun. 2021 Sep 17;570:131-136. doi: 10.1016/j.bbrc.2021.07.047. Epub 2021 Jul 17. Biochem Biophys Res Commun. 2021. PMID: 34280616
-
Centrosome overduplication, chromosomal instability, and human papillomavirus oncoproteins.Environ Mol Mutagen. 2009 Oct;50(8):741-7. doi: 10.1002/em.20478. Environ Mol Mutagen. 2009. PMID: 19326465 Review.
-
Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art.Curr Drug Metab. 2011 Feb;12(2):198-212. doi: 10.2174/138920011795016818. Curr Drug Metab. 2011. PMID: 21395538 Review.
Cited by
-
Functions and dysfunctions of the mammalian centrosome in health, disorders, disease, and aging.Histochem Cell Biol. 2018 Oct;150(4):303-325. doi: 10.1007/s00418-018-1698-1. Epub 2018 Jul 30. Histochem Cell Biol. 2018. PMID: 30062583 Review.
-
A clinical overview of centrosome amplification in human cancers.Int J Biol Sci. 2011;7(8):1122-44. doi: 10.7150/ijbs.7.1122. Epub 2011 Oct 16. Int J Biol Sci. 2011. PMID: 22043171 Free PMC article. Review.
-
Aryl Hydrocarbon Receptor Promotes Liver Polyploidization and Inhibits PI3K, ERK, and Wnt/β-Catenin Signaling.iScience. 2018 Jun 29;4:44-63. doi: 10.1016/j.isci.2018.05.006. Epub 2018 May 15. iScience. 2018. PMID: 30240752 Free PMC article.
-
Defining molecular sensors to assess long-term effects of pesticides on carcinogenesis.Int J Mol Sci. 2014 Sep 25;15(9):17148-61. doi: 10.3390/ijms150917148. Int J Mol Sci. 2014. PMID: 25257533 Free PMC article. Review.
-
The Impact of Centrosome Pathologies on Ovarian Cancer Development and Progression with a Focus on Centrosomes as Therapeutic Target.Adv Exp Med Biol. 2024;1452:37-64. doi: 10.1007/978-3-031-58311-7_3. Adv Exp Med Biol. 2024. PMID: 38805124 Review.
References
-
- Trombino AF, Near RI, Matulka RA, Yang S, Hafer LJ, Toselli PA, Kim DW, Rogers AE, Sonenshein GE, Sherr DH. Expression of the aryl hydrocarbon receptor/transcription factor (AhR) and AhR-regulated CYP1 gene transcripts in a rat model of mammary tumorigenesis. Breast Cancer Res Treat. 2000;63:117–131. doi: 10.1023/A:1006443104670. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

