Anti-tumor activity of N-trimethyl chitosan-encapsulated camptothecin in a mouse melanoma model
- PMID: 20565783
- PMCID: PMC2896352
- DOI: 10.1186/1756-9966-29-76
Anti-tumor activity of N-trimethyl chitosan-encapsulated camptothecin in a mouse melanoma model
Abstract
Background: Camptothecin (CPT) has recently attracted increasing attention as a promising anticancer agent for a variety of tumors. But the clinical application is largely hampered by its extreme water insolubility and unpredictable side effect. It is essential to establish an efficient and safe protocol for the administration of CPT versus melanoma.
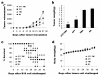
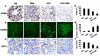
Methods: Camptothecin was encapsulated with N-trimethyl chitosan (CPT-TMC) through microprecipitation and sonication. Its inhibition effect on B16-F10 cell proliferation and induction of apoptosis was evaluated by MTT assay and flow cytometric analysis in vitro. The anti-tumor activity of CPT-TMC was evaluated in C57BL/6 mice bearing B16-F10 melanoma. Tumor volume, tumor weight and survival time were recorded. Assessment of apoptotic cells within tumor tissue was performed by TUNEL assay. Antiangiogenesis and antiproliferation effects of CPT-TMC in vivo were conducted via CD31 and PCNA immunohistochemistry, respectively.
Results: CPT-TMC efficiently inhibited B16-F10 cells proliferation and increased apoptosis in vitro. Experiment group showed significant inhibition compared with free CPT-treated group (81.3% vs. 56.9%) in the growth of B16-F10 melanoma xenografts and prolonged the survival time of the treated mice (P < 0.05). Decreased cell proliferation, increased tumor apoptosis as well as a reduction in angiogenesis were observed.
Conclusions: Our data suggest that N-trimethyl chitosan-encapsulated camptothecin is superior to free CPT by overcoming its insolubility and finally raises the potential of its application in melanoma therapy.
Figures
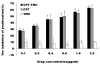

Similar articles
-
The antitumor and antimetastatic effects of N-trimethyl chitosan-encapsulated camptothecin on ovarian cancer with minimal side effects.Oncol Rep. 2010 Oct;24(4):941-8. doi: 10.3892/or.2010.941. Oncol Rep. 2010. PMID: 20811674
-
In vivo antitumor and antimetastatic activities of camptothecin encapsulated with N-trimethyl chitosan in a preclinical mouse model of liver cancer.Cancer Lett. 2010 Nov 1;297(1):56-64. doi: 10.1016/j.canlet.2010.04.024. Epub 2010 May 23. Cancer Lett. 2010. PMID: 20546992
-
Improving Antitumor Activity with N-Trimethyl Chitosan Entrapping Camptothecin in Colon Cancer and Lung Cancer.J Nanosci Nanotechnol. 2015 Sep;15(9):6397-404. doi: 10.1166/jnn.2015.10736. J Nanosci Nanotechnol. 2015. PMID: 26716193
-
The water-insoluble camptothecin analogues: promising drugs for the effective treatment of haematological malignancies.Leuk Res. 1995 Nov;19(11):775-88. doi: 10.1016/0145-2126(95)00048-8. Leuk Res. 1995. PMID: 8551794 Review.
-
Nitrile-Containing Terpyridyl Zn(II)-Coordination Polymer-Based Metallogelators Displaying Helical Structures: Synthesis, Structures, and "Druglike" Action against B16-F10 Melanoma Cells.ACS Appl Mater Interfaces. 2023 May 31;15(21):25098-25109. doi: 10.1021/acsami.2c05338. Epub 2022 Jun 20. ACS Appl Mater Interfaces. 2023. PMID: 35723469 Review.
Cited by
-
Radiobiological Characterization of Canine Malignant Melanoma Cell Lines with Different Types of Ionizing Radiation and Efficacy Evaluation with Cytotoxic Agents.Int J Mol Sci. 2019 Feb 15;20(4):841. doi: 10.3390/ijms20040841. Int J Mol Sci. 2019. PMID: 30781345 Free PMC article.
-
Advances in Chitosan-based Drug Delivery Systems in Melanoma: A Narrative Review.Curr Med Chem. 2024;31(23):3488-3501. doi: 10.2174/0929867330666230518143654. Curr Med Chem. 2024. PMID: 37202890 Review.
-
The synthetic parasite-derived peptide GK1 increases survival in a preclinical mouse melanoma model.Cancer Biother Radiopharm. 2013 Nov;28(9):682-90. doi: 10.1089/cbr.2012.1438. Epub 2013 Jul 10. Cancer Biother Radiopharm. 2013. PMID: 23841709 Free PMC article.
-
Cholesterol depletion by methyl-β-cyclodextrin augments tamoxifen induced cell death by enhancing its uptake in melanoma.Mol Cancer. 2014 Sep 1;13:204. doi: 10.1186/1476-4598-13-204. Mol Cancer. 2014. PMID: 25178635 Free PMC article.
-
Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model.Int J Nanomedicine. 2013;8:4339-50. doi: 10.2147/IJN.S51621. Epub 2013 Nov 5. Int J Nanomedicine. 2013. PMID: 24235831 Free PMC article.
References
-
- Wall ME, Wani MC, Cook EC, Palmer KH, McPhail AT, Sim GA. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata. J Am Chem Soc. 1966;88:3888–3890. doi: 10.1021/ja00968a057. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

