Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study
- PMID: 20565863
- PMCID: PMC2911767
- DOI: 10.1186/cc9074
Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study
Abstract
Introduction: Non-excitable muscle membrane indicates critical illness myopathy (CIM) during early critical illness. We investigated predisposing risk factors for non-excitable muscle membrane at onset of critical illness.
Methods: We performed sequential measurements of muscle membrane excitability after direct muscle stimulation (dmCMAP) in 40 intensive care unit (ICU) patients selected upon a simplified acute physiology (SAPS-II) score >OR= 20 on 3 successive days within 1 week after ICU admission. We then investigated predisposing risk factors, including the insulin-like growth factor (IGF)-system, inflammatory, metabolic and hemodynamic parameters, as well as suspected medical treatment prior to first occurrence of abnormal dmCMAP. Nonparametric analysis of two-factorial longitudinal data and multivariate analysis were used for statistical analysis.
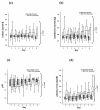
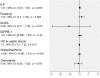
Results: 22 patients showed abnormal muscle membrane excitability during direct muscle stimulation within 7 (5 to 9.25) days after ICU admission. Significant risk factors for the development of impaired muscle membrane excitability in univariate analysis included inflammation, disease severity, catecholamine and sedation requirements, as well as IGF binding protein-1 (IGFBP-I), but did not include either adjunctive hydrocortisone treatment in septic shock, nor administration of neuromuscular blocking agents or aminoglycosides. In multivariate Cox regression analysis, interleukin-6 remained the significant risk factor for the development of impaired muscle membrane excitability (HR 1.006, 95%-CI (1.002 to 1.011), P = 0.002).
Conclusions: Systemic inflammation during early critical illness was found to be the main risk factor for development of CIM during early critical illness. Inflammation-induced impairment of growth-factor mediated insulin sensitivity may be involved in the development of CIM.
Figures
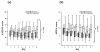
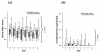
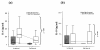
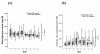
Comment in
-
The role of inflammation in ICU-acquired weakness.Crit Care. 2010;14(4):186. doi: 10.1186/cc9187. Epub 2010 Aug 3. Crit Care. 2010. PMID: 20727229 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

