Proto-oncogene c-erbB2 initiates rat primordial follicle growth via PKC and MAPK pathways
- PMID: 20565902
- PMCID: PMC2903600
- DOI: 10.1186/1477-7827-8-66
Proto-oncogene c-erbB2 initiates rat primordial follicle growth via PKC and MAPK pathways
Abstract
Background: c-erbB2, a proto-oncogene coding epidermal growth factor receptor-like receptor, also as a chemosensitivity/prognosis marker for gynecologic cancer, may be involved in initiation of growth of rat primordial follicles. The aim of the present study is to investigate the role and signal pathway of c-erbB2 in onset of rat primordial follicle development.
Methods: The expression of c-erbB2 mRNA and protein in neonatal ovaries cultured 4 and 8 days with/without epidermal growth factor (EGF) were examined by in situ hybridization, RT-PCR and western blot. The function of c-erbB2 in the primordial folliculogenesis was abolished by small interfering RNA transfection. Furthermore, MAPK inhibitor PD98059 and PKC inhibitor calphostin were used to explore the possible signaling pathway of c-erbB2 in primordial folliculogenesis.
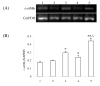
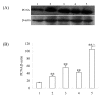
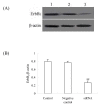
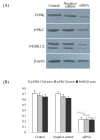
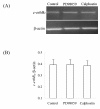
Results: The results showed that c-erbB2 mRNA was expressed in ooplasm and the expression of c-erbB2 decreased after transfection with c-erbB2 siRNA. Treatment with EGF at 50 ng/ml significantly increased c-erbB2 expression and primary and secondary follicle formation in ovaries. However, this augmenting effect was remarkably inhibited by c-erbB2 siRNA transfection. Furthermore, folliculogenesis offset was blocked by calphostin (5 x 10(-4) mmol/L) and PD98059 (5 x 10(-2) mmol/L), but both did not down-regulate c-erbB2 expression. In contrast, the expressions of p-ERK and p-PKC were decreased obviously by c-erbB2 siRNA transfection.
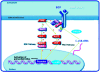
Conclusions: c-erbB2 initiates rat primordial follicle growth via PKC and MAPK pathways, suggesting an important role of c-erbB2 in rat primordial follicle initiation and development.
Figures


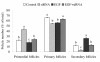
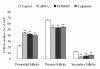
Similar articles
-
The proto-oncogene c-src is involved in primordial follicle activation through the PI3K, PKC and MAPK signaling pathways.Reprod Biol Endocrinol. 2012 Aug 20;10:58. doi: 10.1186/1477-7827-10-58. Reprod Biol Endocrinol. 2012. Retraction in: Reprod Biol Endocrinol. 2024 Oct 31;22(1):134. doi: 10.1186/s12958-024-01305-8. PMID: 22905678 Free PMC article. Retracted.
-
[Role of MAPK and PKC signaling pathways in the regulation of c-erbB₂ in the primordial follicles onset].Sheng Li Xue Bao. 2009 Oct 25;61(5):439-44. Sheng Li Xue Bao. 2009. PMID: 19847364 Chinese.
-
[Roles of proto-oncogene c-erbB2 during the initiation growth of rat primordial follicles].Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2010 May;26(2):165-70. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2010. PMID: 20684269 Chinese.
-
[The expression of proto-oncogene c-erbB₂ and its role in the initiation of primordial follicle growth in rat ovary].Sheng Li Xue Bao. 2009 Oct 25;61(5):424-30. Sheng Li Xue Bao. 2009. PMID: 19847362 Chinese.
-
Epidermal growth factor (EGF) sustains in vitro primordial follicle viability by enhancing stromal cell proliferation via MAPK and PI3K pathways in the prepubertal, but not adult, cat ovary.Biol Reprod. 2014 Apr 25;90(4):86. doi: 10.1095/biolreprod.113.115089. Print 2014 Apr. Biol Reprod. 2014. PMID: 24554736
Cited by
-
The Factors and Pathways Regulating the Activation of Mammalian Primordial Follicles in vivo.Front Cell Dev Biol. 2020 Sep 30;8:575706. doi: 10.3389/fcell.2020.575706. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33102482 Free PMC article. Review.
-
Defining the sister rat mammary tumor cell lines HH-16 cl.2/1 and HH-16.cl.4 as an in vitro cell model for Erbb2.PLoS One. 2012;7(1):e29923. doi: 10.1371/journal.pone.0029923. Epub 2012 Jan 10. PLoS One. 2012. PMID: 22253826 Free PMC article.
-
Untapped Reserves: Controlling Primordial Follicle Growth Activation.Trends Mol Med. 2018 Mar;24(3):319-331. doi: 10.1016/j.molmed.2018.01.008. Epub 2018 Feb 13. Trends Mol Med. 2018. PMID: 29452791 Free PMC article. Review.
-
Mechanisms of acquired resistance to insulin-like growth factor 1 receptor inhibitor in MCF-7 breast cancer cell line.Invest New Drugs. 2013 Apr;31(2):293-303. doi: 10.1007/s10637-012-9855-1. Epub 2012 Jul 25. Invest New Drugs. 2013. PMID: 22828916
-
Regulation of mouse primordial follicle formation by signaling through the PI3K pathway†.Biol Reprod. 2022 Mar 19;106(3):515-525. doi: 10.1093/biolre/ioab204. Biol Reprod. 2022. PMID: 34725674 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

