Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness
- PMID: 20565957
- PMCID: PMC2904286
- DOI: 10.1186/1465-9921-11-82
Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness
Abstract
Background: Pulmonary function has been reported in mice using negative pressure-driven forced expiratory manoeuvres (NPFE) and the forced oscillation technique (FOT). However, both techniques have always been studied using separate cohorts of animals or systems. The objective of this study was to obtain NPFE and FOT measurements at baseline and following bronchoconstriction from a single cohort of mice using a combined system in order to assess both techniques through a refined approach.
Methods: Groups of allergen- or sham-challenged ovalbumin-sensitized mice that were either vehicle (saline) or drug (dexamethasone 1 mg/kg ip)-treated were studied. Surgically prepared animals were connected to an extended flexiVent system (SCIREQ Inc., Montreal, Canada) permitting NPFE and FOT measurements. Lung function was assessed concomitantly by both techniques at baseline and following doubling concentrations of aerosolized methacholine (MCh; 31.25 - 250 mg/ml). The effect of the NPFE manoeuvre on respiratory mechanics was also studied.
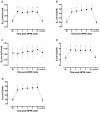
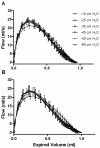
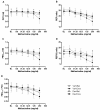
Results: The expected exaggerated MCh airway response of allergic mice and its inhibition by dexamethasone were detected by both techniques. We observed significant changes in FOT parameters at either the highest (Ers, H) or the two highest (Rrs, RN, G) MCh concentrations. The flow-volume (F-V) curves obtained following NPFE manoeuvres demonstrated similar MCh concentration-dependent changes. A dexamethasone-sensitive decrease in the area under the flow-volume curve at the highest MCh concentration was observed in the allergic mice. Two of the four NPFE parameters calculated from the F-V curves, FEV0.1 and FEF50, also captured the expected changes but only at the highest MCh concentration. Normalization to baseline improved the sensitivity of NPFE parameters at detecting the exaggerated MCh airway response of allergic mice but had minimal impact on FOT responses. Finally, the combination with FOT allowed us to demonstrate that NPFE induced persistent airway closure that was reversible by deep lung inflation.
Conclusions: We conclude that FOT and NPFE can be concurrently assessed in the same cohort of animals to determine airway mechanics and expiratory flow limitation during methacholine responses, and that the combination of the two techniques offers a refined control and an improved reproducibility of the NPFE.
Figures
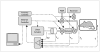
 ) were recorded via precision differential pressure transducers attached respectively to the negative pressure reservoir and the pneumotachograph mounted on the plethysmograph chamber. These signals were collected in addition to the volume displaced by the piston (Vol), the pressure in the cylinder (Pcyl) and the pressure at airway opening (Pao) typically recorded by the flexiVent. PEEP stands for positive end expiratory pressure.
) were recorded via precision differential pressure transducers attached respectively to the negative pressure reservoir and the pneumotachograph mounted on the plethysmograph chamber. These signals were collected in addition to the volume displaced by the piston (Vol), the pressure in the cylinder (Pcyl) and the pressure at airway opening (Pao) typically recorded by the flexiVent. PEEP stands for positive end expiratory pressure.




Similar articles
-
Diagnostic accuracy of methacholine challenge tests assessing airway hyperreactivity in asthmatic patients - a multifunctional approach.Respir Res. 2016 Nov 17;17(1):154. doi: 10.1186/s12931-016-0470-0. Respir Res. 2016. PMID: 27855687 Free PMC article.
-
Methacholine and ovalbumin challenges assessed by forced oscillations and synchrotron lung imaging.Am J Respir Crit Care Med. 2009 Aug 15;180(4):296-303. doi: 10.1164/rccm.200808-1211OC. Epub 2009 May 29. Am J Respir Crit Care Med. 2009. PMID: 19483115
-
Effects of corticosteroid treatment on airway inflammation, mechanics, and hyperpolarized ³He magnetic resonance imaging in an allergic mouse model.J Appl Physiol (1985). 2012 May;112(9):1437-44. doi: 10.1152/japplphysiol.01293.2011. Epub 2012 Jan 12. J Appl Physiol (1985). 2012. Retraction in: J Appl Physiol (1985). 2015 Jun 15;118(12):1561. doi: 10.1152/japplphysiol.zdg-1482-retr.2015. PMID: 22241062 Free PMC article. Retracted.
-
Respiratory impedance measurements for assessment of lung mechanics: focus on asthma.Respir Physiol Neurobiol. 2008 Nov 30;163(1-3):64-73. doi: 10.1016/j.resp.2008.04.015. Epub 2008 Apr 30. Respir Physiol Neurobiol. 2008. PMID: 18579455 Free PMC article. Review.
-
Oscillation mechanics of the respiratory system: applications to lung disease.Crit Rev Biomed Eng. 2011;39(4):337-59. doi: 10.1615/critrevbiomedeng.v39.i4.60. Crit Rev Biomed Eng. 2011. PMID: 22011237 Free PMC article. Review.
Cited by
-
Repeated β2-adrenergic receptor agonist therapy attenuates the response to rescue bronchodilation in a hyperoxic newborn mouse model.Neonatology. 2014;106(2):126-32. doi: 10.1159/000362684. Epub 2014 Jun 20. Neonatology. 2014. PMID: 24969536 Free PMC article.
-
Anisakis pegreffii Extract Induces Airway Inflammation with Airway Remodeling in a Murine Model System.Biomed Res Int. 2021 Sep 17;2021:2522305. doi: 10.1155/2021/2522305. eCollection 2021. Biomed Res Int. 2021. PMID: 34580637 Free PMC article.
-
The Effect of Continuous Positive Airway Pressure in a Mouse Model of Hyperoxic Neonatal Lung Injury.Neonatology. 2016;109(1):6-13. doi: 10.1159/000438818. Epub 2015 Sep 23. Neonatology. 2016. PMID: 26394387 Free PMC article.
-
Lung function measurements in preclinical research: What has been done and where is it headed?Front Physiol. 2023 Mar 22;14:1130096. doi: 10.3389/fphys.2023.1130096. eCollection 2023. Front Physiol. 2023. PMID: 37035677 Free PMC article. Review.
-
Type 1 diabetes contributes to combined pulmonary fibrosis and emphysema in male alpha 1 antitrypsin deficient mice.PLoS One. 2023 Oct 11;18(10):e0291948. doi: 10.1371/journal.pone.0291948. eCollection 2023. PLoS One. 2023. PMID: 37819895 Free PMC article.
References
-
- Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors. Am J Respir Crit Care Med. 2000;161:309–329. - PubMed
-
- Gerald LB, Sockrider MM, Grad R, Bender BG, Boss LP, Galant SP, Gerritsen J, Joseph CL, Kaplan RM, Madden JA, Mangan JM, Redding GJ, Schmidt DK, Schwindt CD, Taggart VS, Wheeler LS, Van Hook KN, Williams PV, Yawn BP, Yuksel B. An official ATS workshop report: issues in screening for asthma in children. Proc Am Thorac Soc. 2007;4:133–141. doi: 10.1513/pats.200604-103ST. - DOI - PubMed
-
- Beydon N, Davis SD, Lombardi E, Allen JL, Arets HGM, Aurora P, Bisgaard H, Davis M, Ducharme FM, Eigen H, Gappa M, Gaultier C, Gustafsson PM, Hall GL, Hantos Z, Healy MJR, Jones MH, Klug B, Lødrup Carlsen KC, McKenzie SA, Marchal F, Mayer OH, Merkus PJFM, Morris MG, Oostveen E, Pillow JJ, Seddon PC, Silverman M, Sly PD, Stocks J, Tepper RS, Vilozni D, Wilson NM. An official American Thoracic Society/European Respiratory Society Statement: Pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. - DOI - PubMed
-
- Hayden MJ, Devadason SG, Sly PD, Wildhaber JH, LeSouef PN. Methacholine responsiveness using the raised volume forced expiration technique in infants. Am J Respir Crit Care Med. 1997;155:1670–1675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

