The function and structure of the cerebrospinal fluid outflow system
- PMID: 20565964
- PMCID: PMC2904716
- DOI: 10.1186/1743-8454-7-9
The function and structure of the cerebrospinal fluid outflow system
Abstract
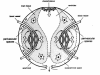
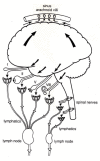
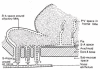
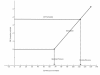
This review traces the development of our understanding of the anatomy and physiological properties of the two systems responsible for the drainage of cerebrospinal fluid (CSF) into the systemic circulation. The roles of the cranial and spinal arachnoid villi (AV) and the lymphatic outflow systems are evaluated as to the dominance of one over the other in various species and degree of animal maturation. The functional capabilities of the total CSF drainage system are presented, with evidence that the duality of the system is supported by the changes in fluid outflow dynamics in human and sub-human primates in hydrocephalus. The review also reconciles the relative importance and alterations of each of the outflow systems in a variety of clinical pathological conditions.
Figures
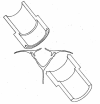
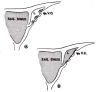
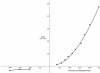
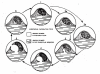
References
-
- Trolard D. Les Lacunes Veineuses de la dura-mere. J de L'anatomie. 1892;38:28–56.
-
- Davson H, Segal MB. Physiology of the Cerebrospinal Fluid and Blood-Brain Barriers. London: CRC Press; 1996.
-
- Key A, Retzius G. Studien in der Anatomie des Nervensystems und des Bindegewebes. Stockholm: Samsin and Wallen; 1875.
LinkOut - more resources
Full Text Sources
Other Literature Sources

