Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine
- PMID: 20565971
- PMCID: PMC2908102
- DOI: 10.1186/1475-2875-9-175
Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine
Abstract
Background: Extensive genetic diversity in vaccine antigens may contribute to the lack of efficacy of blood stage malaria vaccines. Apical membrane antigen-1 (AMA1) is a leading blood stage malaria vaccine candidate with extreme diversity, potentially limiting its efficacy against infection and disease caused by Plasmodium falciparum parasites with diverse forms of AMA1.
Methods: Three hundred Malian children participated in a Phase 2 clinical trial of a bivalent malaria vaccine that found no protective efficacy. The vaccine consists of recombinant AMA1 based on the 3D7 and FVO strains of P. falciparum adjuvanted with aluminum hydroxide (AMA1-C1). The gene encoding AMA1 was sequenced from P. falciparum infections experienced before and after immunization with the study vaccine or a control vaccine. Sequences of ama1 from infections in the malaria vaccine and control groups were compared with regard to similarity to the vaccine antigens using several measures of genetic diversity. Time to infection with parasites carrying AMA1 haplotypes similar to the vaccine strains with respect to immunologically important polymorphisms and the risk of infection with vaccine strain haplotypes were compared.
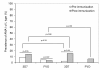
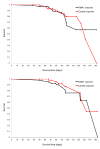
Results: Based on 62 polymorphic AMA1 residues, 186 unique ama1 haplotypes were identified among 315 ama1 sequences that were included in the analysis. Eight infections had ama1 sequences identical to 3D7 while none were identical to FVO. Several measures of genetic diversity showed that ama1 sequences in the malaria vaccine and control groups were comparable both at baseline and during follow up period. Pre- and post-immunization ama1 sequences in both groups all had a similar degree of genetic distance from FVO and 3D7 ama1. No differences were found in the time of first clinical episode or risk of infection with an AMA1 haplotype similar to 3D7 or FVO with respect to a limited set of immunologically important polymorphisms found in the cluster 1 loop of domain I of AMA1.
Conclusion: This Phase 2 trial of a bivalent AMA1 malaria vaccine found no evidence of vaccine selection or strain-specific efficacy, suggesting that the extreme genetic diversity of AMA1 did not account for failure of the vaccine to provide protection.
Figures


Similar articles
-
Molecular basis of allele-specific efficacy of a blood-stage malaria vaccine: vaccine development implications.J Infect Dis. 2013 Feb 1;207(3):511-9. doi: 10.1093/infdis/jis709. Epub 2012 Nov 29. J Infect Dis. 2013. PMID: 23204168 Free PMC article. Clinical Trial.
-
Strain-specific Plasmodium falciparum growth inhibition among Malian children immunized with a blood-stage malaria vaccine.PLoS One. 2017 Mar 10;12(3):e0173294. doi: 10.1371/journal.pone.0173294. eCollection 2017. PLoS One. 2017. PMID: 28282396 Free PMC article. Clinical Trial.
-
Limited antigenic diversity of Plasmodium falciparum apical membrane antigen 1 supports the development of effective multi-allele vaccines.BMC Med. 2014 Oct 16;12:183. doi: 10.1186/s12916-014-0183-5. BMC Med. 2014. PMID: 25319190 Free PMC article.
-
Recombinant protein vaccines against the asexual blood stages of Plasmodium falciparum.Hum Vaccin. 2010 Jan;6(1):39-53. doi: 10.4161/hv.6.1.10712. Epub 2010 Jan 19. Hum Vaccin. 2010. PMID: 20061790 Review.
-
Blood stage vaccines for Plasmodium falciparum: current status and the way forward.Hum Vaccin. 2010 Aug;6(8):627-34. doi: 10.4161/hv.6.8.11446. Hum Vaccin. 2010. PMID: 20519960 Free PMC article. Review.
Cited by
-
Recent advances in recombinant protein-based malaria vaccines.Vaccine. 2015 Dec 22;33(52):7433-43. doi: 10.1016/j.vaccine.2015.09.093. Epub 2015 Oct 11. Vaccine. 2015. PMID: 26458807 Free PMC article. Review.
-
Sequence Analysis of Pvama-1 among Plasmodium Vivax Isolates in Sistan-Baluchistan.Ethiop J Health Sci. 2020 Jul 1;30(4):513-520. doi: 10.4314/ejhs.v30i4.6. Ethiop J Health Sci. 2020. PMID: 33897211 Free PMC article.
-
Our impasse in developing a malaria vaccine.Cell Mol Life Sci. 2011 Apr;68(7):1105-13. doi: 10.1007/s00018-011-0634-5. Epub 2011 Feb 15. Cell Mol Life Sci. 2011. PMID: 21327616 Free PMC article.
-
Malaria Epidemiology at the Clone Level.Trends Parasitol. 2017 Dec;33(12):974-985. doi: 10.1016/j.pt.2017.08.013. Epub 2017 Sep 28. Trends Parasitol. 2017. PMID: 28966050 Free PMC article. Review.
-
Molecular basis of allele-specific efficacy of a blood-stage malaria vaccine: vaccine development implications.J Infect Dis. 2013 Feb 1;207(3):511-9. doi: 10.1093/infdis/jis709. Epub 2012 Nov 29. J Infect Dis. 2013. PMID: 23204168 Free PMC article. Clinical Trial.
References
-
- WHO. World Malaria Report, 2008. http://www.who.int/malaria/wmr2008/malaria2008.pdf
-
- Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, Rare L, Baisor M, Lorry K, Brown GV, Pye D, Irving DO, Smith TA, Beck HP, Alpers MP. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–827. doi: 10.1086/339342. - DOI - PubMed
-
- Alloueche A, Milligan P, Conway DJ, Minder M, Bojang K, Doherty T, Tornieporth N, Cohen J, Greenwood BM. Protective efficacy of the RTS, S/ASO2 Plasmodium falciparum malaria vaccine is not strain specific. Am J Trop Med Hyg. 2003;68:97–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

