Evidence for a role of glutamate as an efferent transmitter in taste buds
- PMID: 20565975
- PMCID: PMC2898831
- DOI: 10.1186/1471-2202-11-77
Evidence for a role of glutamate as an efferent transmitter in taste buds
Abstract
Background: Glutamate has been proposed as a transmitter in the peripheral taste system in addition to its well-documented role as an umami taste stimulus. Evidence for a role as a transmitter includes the presence of ionotropic glutamate receptors in nerve fibers and taste cells, as well as the expression of the glutamate transporter GLAST in Type I taste cells. However, the source and targets of glutamate in lingual tissue are unclear. In the present study, we used molecular, physiological and immunohistochemical methods to investigate the origin of glutamate as well as the targeted receptors in taste buds.
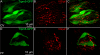
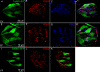
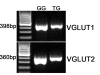
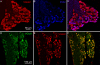
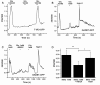
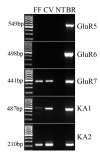
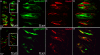
Results: Using molecular and immunohistochemical techniques, we show that the vesicular transporters for glutamate, VGLUT 1 and 2, but not VGLUT3, are expressed in the nerve fibers surrounding taste buds but likely not in taste cells themselves. Further, we show that P2X2, a specific marker for gustatory but not trigeminal fibers, co-localizes with VGLUT2, suggesting the VGLUT-expressing nerve fibers are of gustatory origin. Calcium imaging indicates that GAD67-GFP Type III taste cells, but not T1R3-GFP Type II cells, respond to glutamate at concentrations expected for a glutamate transmitter, and further, that these responses are partially blocked by NBQX, a specific AMPA/Kainate receptor antagonist. RT-PCR and immunohistochemistry confirm the presence of the Kainate receptor GluR7 in Type III taste cells, suggesting it may be a target of glutamate released from gustatory nerve fibers.
Conclusions: Taken together, the results suggest that glutamate may be released from gustatory nerve fibers using a vesicular mechanism to modulate Type III taste cells via GluR7.
Figures

Similar articles
-
Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds.PLoS One. 2012;7(1):e30662. doi: 10.1371/journal.pone.0030662. Epub 2012 Jan 26. PLoS One. 2012. PMID: 22292013 Free PMC article.
-
Knocking out P2X receptors reduces transmitter secretion in taste buds.J Neurosci. 2011 Sep 21;31(38):13654-61. doi: 10.1523/JNEUROSCI.3356-11.2011. J Neurosci. 2011. PMID: 21940456 Free PMC article.
-
Glutamate-induced cobalt uptake elicited by kainate receptors in rat taste bud cells.Chem Senses. 2005 Feb;30(2):137-43. doi: 10.1093/chemse/bji009. Chem Senses. 2005. PMID: 15703333
-
Glutamate: Tastant and Neuromodulator in Taste Buds.Adv Nutr. 2016 Jul 15;7(4):823S-7S. doi: 10.3945/an.115.011304. Print 2016 Jul. Adv Nutr. 2016. PMID: 27422519 Free PMC article. Review.
-
Metabotropic glutamate receptor type 1 in taste tissue.Am J Clin Nutr. 2009 Sep;90(3):743S-746S. doi: 10.3945/ajcn.2009.27462I. Epub 2009 Jul 1. Am J Clin Nutr. 2009. PMID: 19571209 Review.
Cited by
-
The atypical 'hippocampal' glutamate receptor coupled to phospholipase D that controls stretch-sensitivity in primary mechanosensory nerve endings is homomeric purely metabotropic GluK2.Exp Physiol. 2024 Jan;109(1):81-99. doi: 10.1113/EP090761. Epub 2023 Sep 1. Exp Physiol. 2024. PMID: 37656490 Free PMC article. Review.
-
VGLUTs in Peripheral Neurons and the Spinal Cord: Time for a Review.ISRN Neurol. 2013 Nov 20;2013:829753. doi: 10.1155/2013/829753. ISRN Neurol. 2013. PMID: 24349795 Free PMC article. Review.
-
Morphology and chemical characteristics of taste buds associated with P2X3-immunoreactive afferent nerve endings in the rat incisive papilla.J Anat. 2022 Apr;240(4):688-699. doi: 10.1111/joa.13583. Epub 2021 Oct 31. J Anat. 2022. PMID: 34719779 Free PMC article.
-
Adenosine enhances sweet taste through A2B receptors in the taste bud.J Neurosci. 2012 Jan 4;32(1):322-30. doi: 10.1523/JNEUROSCI.4070-11.2012. J Neurosci. 2012. PMID: 22219293 Free PMC article.
-
Diet-induced obesity reduces the responsiveness of the peripheral taste receptor cells.PLoS One. 2013 Nov 13;8(11):e79403. doi: 10.1371/journal.pone.0079403. eCollection 2013. PLoS One. 2013. PMID: 24236129 Free PMC article.
References
-
- Dingledine R, Conn PJ. Peripheral glutamate receptors: molecular biology and role in taste sensation. J Nutr. 2000;130(4S Suppl):1039S–1042S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

