Expression of neurotransmitter transporters for structural and biochemical studies
- PMID: 20566324
- PMCID: PMC2913170
- DOI: 10.1016/j.pep.2010.06.001
Expression of neurotransmitter transporters for structural and biochemical studies
Abstract
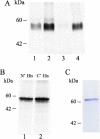
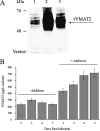
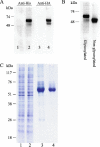
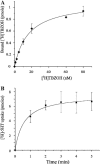
Neurotransmitter transporters play essential roles in the process of neurotransmission. Vesicular neurotransmitter transporters mediate storage inside secretory vesicles in a process that involves the exchange of lumenal H(+) for cytoplasmic transmitter. Retrieval of the neurotransmitter from the synaptic cleft catalyzed by sodium-coupled transporters is critical for the termination of the synaptic actions of the released neurotransmitter. Our current understanding of the mechanism of these transporters is based on functional and biochemical characterization but is lacking high-resolution structural information. Very few structures of membrane transport systems from mammalian origin have been solved to atomic resolution, mainly because of the difficulty in obtaining large amounts of purified protein. Development of high yield heterologous expression systems suitable for mammalian neurotransmitter transporters is essential to enable the production of purified protein for structural studies. Such a system makes possible also the production of mutants that can be used in biochemical and biophysical studies. We describe here a screen for the expression of the vesicular monoamine transporter 2 (VMAT2) in cell-free and baculovirus expression systems and discuss the expression of VMAT2 in other systems as well (bacterial, yeast and mammalian cell lines). After screening and optimization, we achieved high yield (2-2.5 mg/l) expression of functional VMAT2 in insect cells. The system was also used for the expression of three additional plasma membrane neurotransmitter transporters. All were functional and expressed to high levels. Our results demonstrate the advantages of the baculovirus expression system for the expression of mammalian neurotransmitter transporters in a functional state.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Trafficking of vesicular neurotransmitter transporters.Traffic. 2008 Sep;9(9):1425-36. doi: 10.1111/j.1600-0854.2008.00771.x. Epub 2008 May 26. Traffic. 2008. PMID: 18507811 Free PMC article. Review.
-
Elucidating the Mechanism Behind Sodium-Coupled Neurotransmitter Transporters by Reconstitution.Neurochem Res. 2022 Jan;47(1):127-137. doi: 10.1007/s11064-021-03413-y. Epub 2021 Aug 4. Neurochem Res. 2022. PMID: 34347265 Review.
-
How LeuT shapes our understanding of the mechanisms of sodium-coupled neurotransmitter transporters.J Physiol. 2014 Mar 1;592(5):863-9. doi: 10.1113/jphysiol.2013.259051. Epub 2013 Jul 22. J Physiol. 2014. PMID: 23878376 Free PMC article. Review.
-
Neurotransmitter transporters in schistosomes: structure, function and prospects for drug discovery.Parasitol Int. 2013 Dec;62(6):629-38. doi: 10.1016/j.parint.2013.06.003. Epub 2013 Jun 22. Parasitol Int. 2013. PMID: 23800409 Review.
-
Vesicular neurotransmitter transporters: an approach for studying transporters with purified proteins.Physiology (Bethesda). 2013 Jan;28(1):39-50. doi: 10.1152/physiol.00033.2012. Physiology (Bethesda). 2013. PMID: 23280356 Review.
Cited by
-
Identification of molecular hinge points mediating alternating access in the vesicular monoamine transporter VMAT2.Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):E1332-41. doi: 10.1073/pnas.1220497110. Epub 2013 Mar 25. Proc Natl Acad Sci U S A. 2013. PMID: 23530208 Free PMC article.
-
Emulating proton-induced conformational changes in the vesicular monoamine transporter VMAT2 by mutagenesis.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):E7390-E7398. doi: 10.1073/pnas.1605162113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821772 Free PMC article.
-
Structure and mechanism of human vesicular polyamine transporter.Nat Commun. 2025 May 3;16(1):4142. doi: 10.1038/s41467-025-59549-w. Nat Commun. 2025. PMID: 40319071 Free PMC article.
-
SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine.Mol Aspects Med. 2013 Apr-Jun;34(2-3):360-72. doi: 10.1016/j.mam.2012.07.005. Mol Aspects Med. 2013. PMID: 23506877 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

