Escherichia coli cell surface perturbation and disruption induced by antimicrobial peptides BP100 and pepR
- PMID: 20566635
- PMCID: PMC2934620
- DOI: 10.1074/jbc.M110.130955
Escherichia coli cell surface perturbation and disruption induced by antimicrobial peptides BP100 and pepR
Abstract
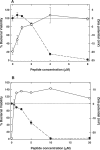
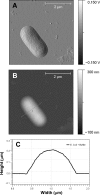
The potential of antimicrobial peptides (AMPs) as an alternative to conventional therapies is well recognized. Insights into the biological and biophysical properties of AMPs are thus key to understanding their mode of action. In this study, the mechanisms adopted by two AMPs in disrupting the gram-negative Escherichia coli bacterial envelope were explored. BP100 is a short cecropin A-melittin hybrid peptide known to inhibit the growth of phytopathogenic gram-negative bacteria. pepR, on the other hand, is a novel AMP derived from the dengue virus capsid protein. Both BP100 and pepR were found to inhibit the growth of E. coli at micromolar concentrations. Zeta potential measurements of E. coli incubated with increasing peptide concentrations allowed for the establishment of a correlation between the minimal inhibitory concentration (MIC) of each AMP and membrane surface charge neutralization. While a neutralization-mediated killing mechanism adopted by either AMP is not necessarily implied, the hypothesis that surface neutralization occurs close to MIC values was confirmed. Atomic force microscopy (AFM) was then employed to visualize the structural effect of the interaction of each AMP with the E. coli cell envelope. At their MICs, BP100 and pepR progressively destroyed the bacterial envelope, with extensive damage already occurring 2 h after peptide addition to the bacteria. A similar effect was observed for each AMP in the concentration-dependent studies. At peptide concentrations below MIC values, only minor disruptions of the bacterial surface occurred.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

