Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with betaENaC-overexpressing mice
- PMID: 20566636
- PMCID: PMC2930694
- DOI: 10.1074/jbc.M110.151803
Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with betaENaC-overexpressing mice
Abstract
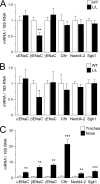
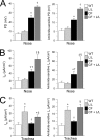
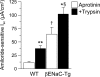
Studies in cystic fibrosis patients and mice overexpressing the epithelial Na(+) channel beta-subunit (betaENaC-Tg) suggest that raised airway Na(+) transport and airway surface liquid (ASL) depletion are central to the pathogenesis of cystic fibrosis lung disease. However, patients or mice with Liddle gain-of-function betaENaC mutations exhibit hypertension but no lung disease. To investigate this apparent paradox, we compared the airway phenotype (nasal versus tracheal) of Liddle with CFTR-null, betaENaC-Tg, and double mutant mice. In mouse nasal epithelium, the region that functionally mimics human airways, high levels of CFTR expression inhibited Liddle epithelial Nat channel (ENaC) hyperfunction. Conversely, in mouse trachea, low levels of CFTR failed to suppress Liddle ENaC hyperfunction. Indeed, Na(+) transport measured in Ussing chambers ("flooded" conditions) was raised in both Liddle and betaENaC-Tg mice. Because enhanced Na(+) transport did not correlate with lung disease in these mutant mice, measurements in tracheal cultures under physiologic "thin film" conditions and in vivo were performed. Regulation of ASL volume and ENaC-mediated Na(+) absorption were intact in Liddle but defective in betaENaC-Tg mice. We conclude that the capacity to regulate Na(+) transport and ASL volume, not absolute Na(+) transport rates in Ussing chambers, is the key physiologic function protecting airways from dehydration-induced lung disease.
Figures



Similar articles
-
Loss of Cftr function exacerbates the phenotype of Na(+) hyperabsorption in murine airways.Am J Physiol Lung Cell Mol Physiol. 2013 Apr 1;304(7):L469-80. doi: 10.1152/ajplung.00150.2012. Epub 2013 Feb 1. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23377346 Free PMC article.
-
Transgenic hCFTR expression fails to correct β-ENaC mouse lung disease.Am J Physiol Lung Cell Mol Physiol. 2012 Jan 15;302(2):L238-47. doi: 10.1152/ajplung.00083.2011. Epub 2011 Oct 14. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22003093 Free PMC article.
-
Ursodeoxycholic acid inhibits ENaC and Na/K pump activity to restore airway surface liquid height in cystic fibrosis bronchial epithelial cells.Steroids. 2019 Nov;151:108461. doi: 10.1016/j.steroids.2019.108461. Epub 2019 Jul 22. Steroids. 2019. PMID: 31344409
-
The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis.Curr Opin Pharmacol. 2018 Dec;43:152-165. doi: 10.1016/j.coph.2018.09.007. Epub 2018 Oct 16. Curr Opin Pharmacol. 2018. PMID: 30340955 Free PMC article. Review.
-
The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis lung disease.Expert Opin Ther Targets. 2018 Aug;22(8):687-701. doi: 10.1080/14728222.2018.1501361. Epub 2018 Jul 26. Expert Opin Ther Targets. 2018. PMID: 30028216 Review.
Cited by
-
Genetic Deletion and Pharmacological Inhibition of PI3K γ Reduces Neutrophilic Airway Inflammation and Lung Damage in Mice with Cystic Fibrosis-Like Lung Disease.Mediators Inflamm. 2015;2015:545417. doi: 10.1155/2015/545417. Epub 2015 Jun 21. Mediators Inflamm. 2015. PMID: 26185363 Free PMC article.
-
SCNN1B regulates the proliferation, migration, and collagen deposition of human lung fibroblasts.In Vitro Cell Dev Biol Anim. 2023 Aug;59(7):479-485. doi: 10.1007/s11626-023-00787-x. Epub 2023 Jul 21. In Vitro Cell Dev Biol Anim. 2023. PMID: 37477776
-
30-Min Exposure to Tobacco Smoke Influences Airway Ion Transport-An In Vitro Study.Curr Oncol. 2023 Jul 22;30(7):7007-7018. doi: 10.3390/curroncol30070508. Curr Oncol. 2023. PMID: 37504368 Free PMC article.
-
Acute effects of cigarette smoke extract on alveolar epithelial sodium channel activity and lung fluid clearance.Am J Respir Cell Mol Biol. 2013 Aug;49(2):251-9. doi: 10.1165/rcmb.2012-0234OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526224 Free PMC article.
-
Loss of Cftr function exacerbates the phenotype of Na(+) hyperabsorption in murine airways.Am J Physiol Lung Cell Mol Physiol. 2013 Apr 1;304(7):L469-80. doi: 10.1152/ajplung.00150.2012. Epub 2013 Feb 1. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23377346 Free PMC article.
References
-
- Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., Horisberger J. D., Rossier B. C. (1994) Nature 367, 463–467 - PubMed
-
- Rossier B. C., Pradervand S., Schild L., Hummler E. (2002) Annu. Rev. Physiol. 64, 877–897 - PubMed
-
- Kunzelmann K., Mall M. (2002) Physiol. Rev. 82, 245–289 - PubMed
-
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. (1983) Science 221, 1067–1070 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

