Nicotinic receptor subunit alpha5 modifies assembly, up-regulation, and response to pro-inflammatory cytokines
- PMID: 20566638
- PMCID: PMC2924004
- DOI: 10.1074/jbc.M110.105346
Nicotinic receptor subunit alpha5 modifies assembly, up-regulation, and response to pro-inflammatory cytokines
Abstract
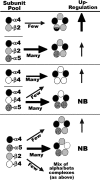
In the mammalian brain high affinity nicotine-binding sites are composed of at least the alpha4 and beta2 subunits. Additional nicotinic acetylcholine receptor subunits that are often co-expressed with alpha4+beta2 include alpha5. The introduction of alpha5 into 293 cells expressing alpha4+beta2 strongly favors assembly of alpha4+alpha5+beta2 receptors, increases constitutive ligand binding density as measured using [(3)H]epibatidine, but reduces the magnitude of up-regulation in response to chronic nicotine. In contrast, when beta4 is substituted for beta2, alpha5 interferes with the assembly of these receptors, demonstrating an important role for the beta subunit in this process. When cells co-express alpha4+alpha5+beta2+beta4, over 50% of the subunit associations include all four subunits, but they fail to be detected using [(3)H]epibatidine binding. However, complexes of alpha4+alpha5+beta2 do preferentially emerge from these subunit mixtures, and these mixtures bind ligand. In previous studies of alpha4+beta2+beta4 co-expression by 293 cells, the inflammatory cytokines IL-1beta and TNFalpha influenced the outcome of receptor assembly (Gahring, L. C., Days, E. L., Kaasch, T., González de Mendoza, M., Owen, L., Persiyanov, K., and Rogers, S. W. (2005) J. Neuroimmunol. 166, 88-101). When alpha5 is included in this subunit mixture, and cells are exposed to either inflammatory cytokine, subunit association is no longer altered. These findings suggest that alpha5 is an influential modulator of alpha4+beta2 nicotinic acetylcholine receptor assembly and stabilizes their expression in response to fluctuations in external conditions.
Figures





Similar articles
-
Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits.J Biol Chem. 1996 Jul 26;271(30):17656-65. doi: 10.1074/jbc.271.30.17656. J Biol Chem. 1996. PMID: 8663494
-
Nicotinic receptor subtypes in the developing chick brain: appearance of a species containing the alpha4, beta2, and alpha5 gene products.Mol Pharmacol. 1998 Mar;53(3):392-401. doi: 10.1124/mol.53.3.392. Mol Pharmacol. 1998. PMID: 9495803
-
Probing the non-canonical interface for agonist interaction with an α5 containing nicotinic acetylcholine receptor.Neuropharmacology. 2014 Feb;77:342-9. doi: 10.1016/j.neuropharm.2013.09.028. Epub 2013 Oct 18. Neuropharmacology. 2014. PMID: 24144909 Free PMC article.
-
In vivo chronic nicotine exposure differentially and reversibly affects upregulation and stoichiometry of α4β2 nicotinic receptors in cortex and thalamus.Neuropharmacology. 2016 Sep;108:324-31. doi: 10.1016/j.neuropharm.2016.04.048. Epub 2016 May 6. Neuropharmacology. 2016. PMID: 27157710
-
The role of nicotinic receptors in B-lymphocyte development and activation.Life Sci. 2007 May 30;80(24-25):2334-6. doi: 10.1016/j.lfs.2007.02.005. Epub 2007 Feb 20. Life Sci. 2007. PMID: 17363009 Review.
Cited by
-
Chrna5 genotype determines the long-lasting effects of developmental in vivo nicotine exposure on prefrontal attention circuitry.Neuropharmacology. 2014 Feb;77:145-55. doi: 10.1016/j.neuropharm.2013.09.003. Epub 2013 Sep 18. Neuropharmacology. 2014. PMID: 24055499 Free PMC article.
-
Nicotine enhances excitability of medial habenular neurons via facilitation of neurokinin signaling.J Neurosci. 2014 Mar 19;34(12):4273-84. doi: 10.1523/JNEUROSCI.2736-13.2014. J Neurosci. 2014. PMID: 24647947 Free PMC article.
-
Upregulation of Nicotinic Acetylcholine Receptor alph4+beta2 through a Ligand-Independent PI3Kbeta Mechanism That Is Enhanced by TNFalpha and the Jak2/p38Mapk Pathways.PLoS One. 2015 Nov 30;10(11):e0143319. doi: 10.1371/journal.pone.0143319. eCollection 2015. PLoS One. 2015. PMID: 26619345 Free PMC article.
-
Function of human α3β4α5 nicotinic acetylcholine receptors is reduced by the α5(D398N) variant.J Biol Chem. 2012 Jul 20;287(30):25151-62. doi: 10.1074/jbc.M112.379339. Epub 2012 Jun 4. J Biol Chem. 2012. PMID: 22665477 Free PMC article.
-
Alcohol-Induced Increases in Inflammatory Cytokines Are Attenuated by Nicotine in Region-Selective Manner in Male Rats.J Drug Alcohol Res. 2017;6:236036. doi: 10.4303/jdar/236036. Epub 2017 Sep 16. J Drug Alcohol Res. 2017. PMID: 29416901 Free PMC article.
References
-
- Schwartz R. D., Kellar K. J. (1985) J. Neurochem. 45, 427–433 - PubMed
-
- Marks M. J., Stitzel J. A., Collins A. C. (1985) J. Pharmacol. Exp. Ther. 235, 619–628 - PubMed
-
- Flores C. M., Rogers S. W., Pabreza L. A., Wolfe B. B., Kellar K. J. (1992) Mol. Pharmacol. 41, 31–37 - PubMed
-
- Picciotto M. R., Zoli M., Changeux J. P. (1999) Nicotine Tob. Res. Suppl. 2, S121–S125 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

