Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury
- PMID: 20566639
- PMCID: PMC2924062
- DOI: 10.1074/jbc.M110.147439
Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury
Abstract
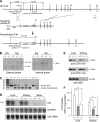
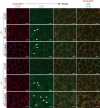
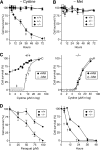
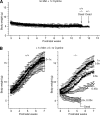
Cysteine is considered a nonessential amino acid in mammals as it is synthesized from methionine via trans-sulfuration. However, premature infants or patients with hepatic failure may require dietary cysteine due to a lack of cystathionine gamma-lyase (CTH), a key trans-sulfuration enzyme. Here, we generated CTH-deficient (Cth(-/-)) mice as an animal model of cystathioninemia/cystathioninuria. Cth(-/-) mice developed normally in general but displayed hypercystathioninemia/hyperhomocysteinemia though not hypermethioninemia. When fed a low cyst(e)ine diet, Cth(-/-) mice showed acute skeletal muscle atrophy (myopathy) accompanied by enhanced gene expression of asparagine synthetase and reduced contents of glutathione in livers and skeletal muscles, and intracellular accumulation of LC3 and p62 in skeletal myofibers; they finally died of severe paralysis of the extremities. Cth(-/-) hepatocytes required cystine in a culture medium and showed greater sensitivity to oxidative stress. Cth(-/-) mice exhibited systemic vulnerability to oxidative injury, which became more prominent when they were fed the low cyst(e)ine diet. These results reveal novel roles of trans-sulfuration previously unrecognized in mice lacking another trans-sulfuration enzyme cystathionine beta-synthase (Cbs(-/-)). Because Cbs(-/-) mice display hyperhomocysteinemia and hypermethioninemia, our results raise questions against the homocysteine-based etiology of CBS deficiency and the current newborn screening for homocysteinemia using Guthrie's method, which detects hypermethioninemia.
Figures



Similar articles
-
Neutral aminoaciduria in cystathionine β-synthase-deficient mice; an animal model of homocystinuria.Am J Physiol Renal Physiol. 2014 Jun 15;306(12):F1462-76. doi: 10.1152/ajprenal.00623.2013. Epub 2014 Apr 23. Am J Physiol Renal Physiol. 2014. PMID: 24761004
-
Methionine excess in diet induces acute lethal hepatitis in mice lacking cystathionine γ-lyase, an animal model of cystathioninuria.Free Radic Biol Med. 2012 May 1;52(9):1716-26. doi: 10.1016/j.freeradbiomed.2012.02.033. Epub 2012 Mar 3. Free Radic Biol Med. 2012. PMID: 22387178
-
Preeclampsia-Like Features and Partial Lactation Failure in Mice Lacking Cystathionine γ-Lyase-An Animal Model of Cystathioninuria.Int J Mol Sci. 2019 Jul 17;20(14):3507. doi: 10.3390/ijms20143507. Int J Mol Sci. 2019. PMID: 31319489 Free PMC article.
-
[Cystathionine γ-lyase].Postepy Hig Med Dosw (Online). 2014 Jan 15;68:1-9. doi: 10.5604/17322693.1085372. Postepy Hig Med Dosw (Online). 2014. PMID: 24491890 Review. Polish.
-
Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-β- synthase (CBS) and cystathionine-γ-lyase (CSE).Inflamm Allergy Drug Targets. 2011 Apr;10(2):85-91. doi: 10.2174/187152811794776286. Inflamm Allergy Drug Targets. 2011. PMID: 21275900 Review.
Cited by
-
Modes of physiologic H2S signaling in the brain and peripheral tissues.Antioxid Redox Signal. 2015 Feb 10;22(5):411-23. doi: 10.1089/ars.2014.5917. Epub 2014 May 9. Antioxid Redox Signal. 2015. PMID: 24684551 Free PMC article. Review.
-
The Gasotransmitter Hydrogen Sulfide (H2S) Prevents Pathologic Calcification (PC) in Cartilage.Antioxidants (Basel). 2021 Sep 8;10(9):1433. doi: 10.3390/antiox10091433. Antioxidants (Basel). 2021. PMID: 34573065 Free PMC article.
-
Identification of Proteins Interacting with Cytoplasmic High-Mobility Group Box 1 during the Hepatocellular Response to Ischemia Reperfusion Injury.Int J Mol Sci. 2017 Jan 16;18(1):167. doi: 10.3390/ijms18010167. Int J Mol Sci. 2017. PMID: 28275217 Free PMC article.
-
PLP-dependent H(2)S biogenesis.Biochim Biophys Acta. 2011 Nov;1814(11):1518-27. doi: 10.1016/j.bbapap.2011.02.004. Epub 2011 Feb 17. Biochim Biophys Acta. 2011. PMID: 21315854 Free PMC article. Review.
-
Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels.Circ Res. 2011 Nov 11;109(11):1259-68. doi: 10.1161/CIRCRESAHA.111.240242. Epub 2011 Oct 6. Circ Res. 2011. PMID: 21980127 Free PMC article.
References
-
- Finkelstein J. D. (2000) Semin. Thromb. Hemost. 26, 219–225 - PubMed
-
- Stipanuk M. H. (2004) Annu. Rev. Nutr. 24, 539–577 - PubMed
-
- Sturman J. A., Gaull G., Raiha N. C. (1970) Science 169, 74–76 - PubMed
-
- Zlotkin S. H., Anderson G. H. (1982) Pediatr. Res. 16, 65–68 - PubMed
-
- Viña J., Vento M., García-Sala F., Puertes I. R., Gascó E., Sastre J., Asensi M., Pallardó F. V. (1995) Am. J. Clin. Nutr. 61, 1067–1069 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

