Predicting cardiomyopathic phenotypes by altering Ca2+ affinity of cardiac troponin C
- PMID: 20566645
- PMCID: PMC2934646
- DOI: 10.1074/jbc.M110.112326
Predicting cardiomyopathic phenotypes by altering Ca2+ affinity of cardiac troponin C
Abstract
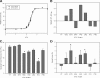
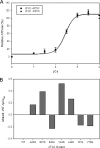
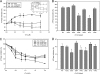
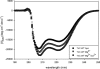
Cardiac diseases associated with mutations in troponin subunits include hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), and restrictive cardiomyopathy (RCM). Altered calcium handling in these diseases is evidenced by changes in the Ca(2+) sensitivity of contraction. Mutations in the Ca(2+) sensor, troponin C (TnC), were generated to increase/decrease the Ca(2+) sensitivity of cardiac skinned fibers to create the characteristic effects of DCM, HCM, and RCM. We also used a reconstituted assay to determine the mutation effects on ATPase activation and inhibition. One mutant (A23Q) was found with HCM-like properties (increased Ca(2+) sensitivity of force and normal levels of ATPase inhibition). Three mutants (S37G, V44Q, and L48Q) were identified with RCM-like properties (a large increase in Ca(2+) sensitivity, partial loss of ATPase inhibition, and increased basal force). Two mutations were identified (E40A and I61Q) with DCM properties (decreased Ca(2+) sensitivity, maximal force recovery, and activation of the ATPase at high [Ca(2+)]). Steady-state fluorescence was utilized to assess Ca(2+) affinity in isolated cardiac (c)TnCs containing F27W and did not necessarily mirror the fiber Ca(2+) sensitivity. Circular dichroism of mutant cTnCs revealed a trend where increased alpha-helical content correlated with increased Ca(2+) sensitivity in skinned fibers and vice versa. The main findings from this study were as follows: 1) cTnC mutants demonstrated distinct functional phenotypes reminiscent of bona fide HCM, RCM, and DCM mutations; 2) a region in cTnC associated with increased Ca(2+) sensitivity in skinned fibers was identified; and 3) the F27W reporter mutation affected Ca(2+) sensitivity, maximal force, and ATPase activation of some mutants.
Figures

References
-
- Pollesello P., Ovaska M., Kaivola J., Tilgmann C., Lundström K., Kalkkinen N., Ulmanen I., Nissinen E., Taskinen J. (1994) J. Biol. Chem. 269, 28584–28590 - PubMed
-
- Sorsa T., Heikkinen S., Abbott M. B., Abusamhadneh E., Laakso T., Tilgmann C., Serimaa R., Annila A., Rosevear P. R., Drakenberg T., Pollesello P., Kilpelainen I. (2001) J. Biol. Chem. 276, 9337–9343 - PubMed
-
- el-Saleh S. C., Solaro R. J. (1987) J. Biol. Chem. 262, 17240–17246 - PubMed
-
- Chandra M., Rundell V. L., Tardiff J. C., Leinwand L. A., De Tombe P. P., Solaro R. J. (2001) Am. J. Physiol. Heart Circ. Physiol. 280, H705–H713 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

