Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle
- PMID: 20566647
- PMCID: PMC2919128
- DOI: 10.1074/jbc.M110.104976
Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle
Abstract
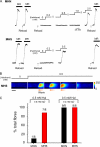
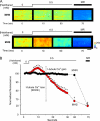
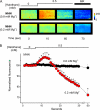
In malignant hyperthermia (MH), mutations in RyR1 underlie direct activation of the channel by volatile anesthetics, leading to muscle contracture and a life-threatening increase in core body temperature. The aim of the present study was to establish whether the associated depletion of sarcoplasmic reticulum (SR) Ca(2+) triggers sarcolemmal Ca(2+) influx via store-operated Ca(2+) entry (SOCE). Samples of vastus medialis muscle were obtained from patients undergoing assessment for MH susceptibility using the in vitro contracture test. Single fibers were mechanically skinned, and confocal microscopy was used to detect changes in [Ca(2+)] either within the resealed t-system ([Ca(2+)](t-sys)) or within the cytosol. In normal fibers, halothane (0.5 mM) failed to initiate SR Ca(2+) release or Ca(2+)(t-sys) depletion. However, in MH-susceptible (MHS) fibers, halothane induced both SR Ca(2+) release and Ca(2+)(t-sys) depletion, consistent with SOCE. In some MHS fibers, halothane-induced SR Ca(2+) release took the form of a propagated wave, which was temporally coupled to a wave of Ca(2+)(t-sys) depletion. SOCE was potently inhibited by "extracellular" application of a STIM1 antibody trapped within the t-system but not when the antibody was denatured by heating. In conclusion, (i) in human MHS muscle, SR Ca(2+) depletion induced by a level of volatile anesthetic within the clinical range is sufficient to induce SOCE, which is tightly coupled to SR Ca(2+) release; (ii) sarcolemmal STIM1 has an important role in regulating SOCE; and (iii) sustained SOCE from an effectively infinite extracellular Ca(2+) pool may contribute to the maintained rise in cytosolic [Ca(2+)] that underlies MH.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

