Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic beta-cell function
- PMID: 20566664
- PMCID: PMC2927943
- DOI: 10.2337/db09-1552
Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic beta-cell function
Erratum in
- Diabetes. 2010 Dec;59(12):3257
Abstract
Objective: Pancreatic-derived factor (PANDER, FAM3B) is a pancreatic islet-specific cytokine-like protein that is secreted from beta-cells upon glucose stimulation. The biological function of PANDER is unknown, and to address this we generated and characterized a PANDER knockout mouse.
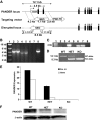
Research design and methods: To generate the PANDER knockout mouse, the PANDER gene was disrupted and its expression was inhibited by homologous recombination via replacement of the first two exons, secretion signal peptide and transcriptional start site, with the neomycin gene. PANDER(-/-) mice were then phenotyped by a number of in vitro and in vivo tests to evaluate potential effects on glucose regulation, insulin sensitivity, and beta-cell morphology and function.
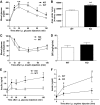
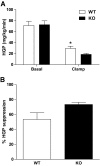
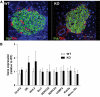
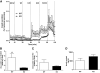
Results: Glucose tolerance tests demonstrated significantly higher blood glucose levels in PANDER(-/-) versus wild-type male mice. To identify the mechanism of the glucose intolerance, insulin sensitivity and pancreatic beta-cell function were examined. Hyperinsulinemic-euglycemic clamps and insulin tolerance testing showed similar insulin sensitivity for both the PANDER(-/-) and wild-type mice. The in vivo insulin response following intraperitoneal glucose injection surprisingly produced significantly higher insulin levels in the PANDER(-/-) mice, whereas insulin release was blunted with arginine administration. Islet perifusion and calcium imaging studies showed abnormal responses of the PANDER(-/-) islets to glucose stimulation. In contrast, neither islet architecture nor insulin content was impacted by the loss of PANDER. Interestingly, the elevated insulin levels identified in vivo were attributed to decreased hepatic insulin clearance in the PANDER(-/-) islets. Taken together, these results demonstrated decreased pancreatic beta-cell function in the PANDER(-/-) mouse.
Conclusions: These results support a potential role of PANDER in the pancreatic beta-cell for regulation or facilitation of insulin secretion.
Figures

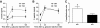
References
-
- Zhu Y, Xu G, Patel A, McLaughlin MM, Silverman C, Knecht K, Sweitzer S, Li X, McDonnell P, Mirabile R, Zimmerman D, Boyce R, Tierney LA, Hu E, Livi GP, Wolf B, Abdel-Meguid SS, Rose GD, Aurora R, Hensley P, Briggs M, Young PR. Cloning, expression, and initial characterization of a novel cytokine-like gene family. Genomics 2002;80:144–150 - PubMed
-
- Cao X, Gao Z, Robert CE, Greene S, Xu G, Xu W, Bell E, Campbell D, Zhu Y, Young R, Trucco M, Markmann JF, Naji A, Wolf BA. Pancreatic-derived factor (FAM3B), a novel islet cytokine, induces apoptosis of insulin-secreting beta-cells. Diabetes 2003;52:2296–2303 - PubMed
-
- Cao X, Yang J, Burkhardt BR, Gao Z, Wong RK, Greene SR, Wu J, Wolf BA. Effects of overexpression of pancreatic derived factor (FAM3B) in isolated mouse islets and insulin-secreting betaTC3 cells. Am J Physiol Endocrinol Metab 2005;289:E543–E550 - PubMed
-
- Yang J, Gao Z, Robert CE, Burkhardt BR, Gaweska H, Wagner A, Wu J, Greene SR, Young RA, Wolf BA: Structure-function studies of PANDER, an islet specific cytokine inducing cell death of insulin-secreting beta cells. Biochemistry 2005;44:11342–11352 - PubMed
-
- Burkhardt BR, Greene SR, White P, Wong RK, Brestelli JE, Yang J, Robert CE, Brusko TM, Wasserfall CH, Wu J, Atkinson MA, Gao Z, Kaestner KH, Wolf BA. PANDER-induced cell-death genetic networks in islets reveal central role for caspase-3 and cyclin-dependent kinase inhibitor 1A (p21). Gene 2006;369:134–141 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

