Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine
- PMID: 20566739
- PMCID: PMC2913341
- DOI: 10.2353/ajpath.2010.090998
Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine
Abstract
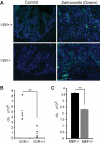
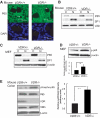
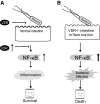
Vitamin D receptor (VDR) plays an essential role in gastrointestinal inflammation. Most investigations have focused on the immune response; however, how bacteria regulate VDR and how VDR modulates the nuclear factor (NF)-kappaB pathway in intestinal epithelial cells remain unexplored. This study investigated the effects of VDR ablation on NF-kappaB activation in intestinal epithelia and the role of enteric bacteria on VDR expression. We found that VDR(-/-) mice exhibited a pro-inflammatory bias. After Salmonella infection, VDR(-/-) mice had increased bacterial burden and mortality. Serum interleukin-6 in noninfected VDR(+/+) mice was undetectable, but was easily detectable in VDR(-/-) mice. NF-kappaB p65 formed a complex with VDR in noninfected wild-type mouse intestine. In contrast, deletion of VDR abolished VDR/P65 binding. P65 nuclear translocation occurred in colonic epithelial cells of untreated VDR(-/-) mice. VDR deletion also elevated NF-kappaB activity in intestinal epithelia. VDR was localized to the surface epithelia of germ-free mice, but to crypt epithelial cells in conventionalized mice. VDR expression, distribution, transcriptional activity, and target genes were regulated by Salmonella stimulation, independent of 1,25-dihydroxyvitamin D3. Our study demonstrates that commensal and pathogenic bacteria directly regulate colonic epithelial VDR expression and location in vivo. VDR negatively regulates bacterial-induced intestinal NF-kappaB activation and attenuates response to infection. Therefore, VDR is an important contributor to intestinal homeostasis and host protection from bacterial invasion and infection.
Figures





References
-
- Haussler MR, Whitfield GK, Haussler CA, Hsieh J-C, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone and Mineral Research. 1998;13:325–349. - PubMed
-
- Demay MB. Mechanism of vitamin D receptor action. Ann NY Acad Sci. 2006;1068:204–213. - PubMed
-
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

