Role of ES cell-expressed Ras (ERas) in tumorigenicity of gastric cancer
- PMID: 20566745
- PMCID: PMC2913346
- DOI: 10.2353/ajpath.2010.091056
Role of ES cell-expressed Ras (ERas) in tumorigenicity of gastric cancer
Abstract
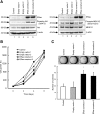
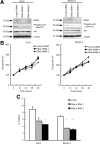
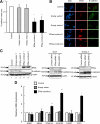
ERas, a unique member of the Ras family, was initially found only in embryonic stem (ES) cells, where it plays a crucial role in the transformation of transplanted ES cells to teratomas. ERas is involved in ES cell survival, and unlike other Ras family members, is constitutively active without any mutations. The aim of this study was to investigate the expression and role of ERas in human gastric cancer. To test whether ERas played a significant role in human cancer cells, we examined its expression and function in gastric cancer. ERas was expressed in gastric cancer cell lines at different levels. Induction of ERas expression activated the phosphatidylinositol 3 kinase (PI3K)/Akt axis and then enhanced anchorage-independent growth and ERas knockdown by siRNA suppressed cell invasion. Immunohistochemical analyses revealed that ERas was expressed in 38.7% (55/142) of human gastric carcinoma tissues, and its expression was significantly associated with metastasis to the liver (P < 0.0001) and lymph nodes (P < 0.05). ERas up-regulated transcription regulatory factors including ZFHX1A, ZFHX1B, and TCF3, which repress E-cadherin. These data suggest that ERas is activated in a significant population of gastric cancer, where it may play a crucial role in gastric cancer cell survival and metastases to liver via down-regulation of E-cadherin.
Figures
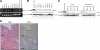

Similar articles
-
Role of the ERas gene in gastric cancer cells.Oncol Rep. 2013 Jul;30(1):50-6. doi: 10.3892/or.2013.2417. Epub 2013 Apr 23. Oncol Rep. 2013. PMID: 23612786 Free PMC article.
-
Expression of ERas oncogene in gastric carcinoma.Anticancer Res. 2009 Jun;29(6):2189-93. Anticancer Res. 2009. PMID: 19528480
-
Epigenetic regulation of the embryonic oncogene ERas in gastric cancer cells.Int J Oncol. 2009 Nov;35(5):997-1003. doi: 10.3892/ijo_00000414. Int J Oncol. 2009. PMID: 19787253
-
ERas is expressed in primate embryonic stem cells but not related to tumorigenesis.Cell Transplant. 2009;18(4):381-9. doi: 10.3727/096368909788809794. Cell Transplant. 2009. PMID: 19622226
-
Role of the phosphoinositide 3-kinase pathway in mouse embryonic stem (ES) cells.Biochem Soc Trans. 2005 Dec;33(Pt 6):1522-5. doi: 10.1042/BST0331522. Biochem Soc Trans. 2005. PMID: 16246160 Review.
Cited by
-
ERAS, a Member of the Ras Superfamily, Acts as an Oncoprotein in the Mammary Gland.Cancers (Basel). 2021 Nov 8;13(21):5588. doi: 10.3390/cancers13215588. Cancers (Basel). 2021. PMID: 34771750 Free PMC article.
-
The roles of ERAS during cell lineage specification of mouse early embryonic development.Open Biol. 2015 Aug;5(8):150092. doi: 10.1098/rsob.150092. Open Biol. 2015. PMID: 26269429 Free PMC article.
-
Putting the Pieces Together: Completing the Mechanism of Action Jigsaw for Sipuleucel-T.J Natl Cancer Inst. 2020 Jun 1;112(6):562-573. doi: 10.1093/jnci/djaa021. J Natl Cancer Inst. 2020. PMID: 32145020 Free PMC article. Review.
-
ERAS Is Constitutively Expressed in the Tissues of Adult Horses and May Be a Key Player in Basal Autophagy.Front Vet Sci. 2022 May 24;9:818294. doi: 10.3389/fvets.2022.818294. eCollection 2022. Front Vet Sci. 2022. PMID: 35685342 Free PMC article.
-
Functional genomics identifies drivers of medulloblastoma dissemination.Cancer Res. 2012 Oct 1;72(19):4944-53. doi: 10.1158/0008-5472.CAN-12-1629. Epub 2012 Aug 8. Cancer Res. 2012. PMID: 22875024 Free PMC article.
References
-
- Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. - PubMed
-
- Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–678. - PubMed
-
- Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. - PubMed
-
- Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–554. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

