Abnormalities in mitochondrial structure in cells from patients with bipolar disorder
- PMID: 20566748
- PMCID: PMC2913344
- DOI: 10.2353/ajpath.2010.081068
Abnormalities in mitochondrial structure in cells from patients with bipolar disorder
Abstract
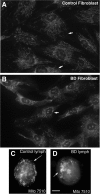
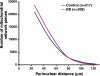
Postmortem, genetic, brain imaging, and peripheral cell studies all support decreased mitochondrial activity as a factor in the manifestation of Bipolar Disorder (BD). Because abnormal mitochondrial morphology is often linked to altered energy metabolism, we investigated whether changes in mitochondrial structure were present in brain and peripheral cells of patients with BD. Mitochondria from patients with BD exhibited size and distributional abnormalities compared with psychiatrically-healthy age-matched controls. Specifically, in brain, individual mitochondria profiles had significantly smaller areas, on average, in BD samples (P = 0.03). In peripheral cells, mitochondria in BD samples were concentrated proportionately more within the perinuclear region than in distal processes (P = 0.0008). These mitochondrial changes did not appear to be correlated with exposure to lithium. Also, these abnormalities in brain and peripheral cells were independent of substantial changes in the actin or tubulin cytoskeleton with which mitochondria interact. The observed changes in mitochondrial size and distribution may be linked to energy deficits and, therefore, may have consequences for cell plasticity, resilience, and survival in patients with BD, especially in brain, which has a high-energy requirement. The findings may have implications for diagnosis, if they are specific to BD, and for treatment, if they provide clues as to the underlying pathophysiology of BD.
Figures




References
-
- Berns GS, Nemeroff CB. The neurobiology of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:76–84. - PubMed
-
- Rajkowska G. Cell pathology in bipolar disorder. Bipolar Disord. 2002;4:105–116. - PubMed
-
- Quiroz JA, Gray NA, Kato T, Manji HK. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:2551–2565. - PubMed
-
- Kato T, Stine OC, McMahon FJ, Crowe RR. Increased levels of a mitochondrial DNA deletion in the brain of patients with bipolar disorder. Biol Psychiatry. 1997;42:871–875. - PubMed
-
- Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

