Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota
- PMID: 20566854
- PMCID: PMC2901479
- DOI: 10.1073/pnas.0909122107
Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota
Abstract
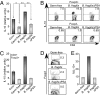
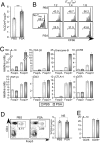
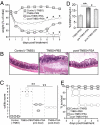
To maintain intestinal health, the immune system must faithfully respond to antigens from pathogenic microbes while limiting reactions to self-molecules. The gastrointestinal tract represents a unique challenge to the immune system, as it is permanently colonized by a diverse amalgam of bacterial phylotypes producing multitudes of foreign microbial products. Evidence from human and animal studies indicates that inflammatory bowel disease results from uncontrolled inflammation to the intestinal microbiota. However, molecular mechanisms that actively promote mucosal tolerance to the microbiota remain unknown. We report herein that a prominent human commensal, Bacteroides fragilis, directs the development of Foxp3(+) regulatory T cells (Tregs) with a unique "inducible" genetic signature. Monocolonization of germ-free animals with B. fragilis increases the suppressive capacity of Tregs and induces anti-inflammatory cytokine production exclusively from Foxp3(+) T cells in the gut. We show that the immunomodulatory molecule, polysaccharide A (PSA), of B. fragilis mediates the conversion of CD4(+) T cells into Foxp3(+) Treg cells that produce IL-10 during commensal colonization. Functional Foxp3(+) Treg cells are also produced by PSA during intestinal inflammation, and Toll-like receptor 2 signaling is required for both Treg induction and IL-10 expression. Most significantly, we show that PSA is not only able to prevent, but also cure experimental colitis in animals. Our results therefore demonstrate that B. fragilis co-opts the Treg lineage differentiation pathway in the gut to actively induce mucosal tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Symbiosis: A helping hand from Bacteroides fragilis.Nat Rev Microbiol. 2010 Aug;8(8):534. doi: 10.1038/nrmicro2413. Nat Rev Microbiol. 2010. PMID: 20665956 No abstract available.
-
Regulatory T cells: A helping hand from Bacteroides fragilis.Nat Rev Immunol. 2010 Aug;10(8):539. doi: 10.1038/nri2827. Nat Rev Immunol. 2010. PMID: 20677358 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

