Identification of RL-TGR, a coreceptor involved in aversive chemical signaling
- PMID: 20566865
- PMCID: PMC2901457
- DOI: 10.1073/pnas.1000343107
Identification of RL-TGR, a coreceptor involved in aversive chemical signaling
Abstract
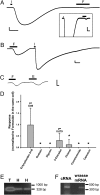
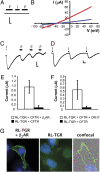
Chemical signaling plays an important role in predator-prey interactions and feeding dynamics. Like other organisms that are sessile or slow moving, some marine sponges contain aversive compounds that defend these organisms from predation. We sought to identify and characterize a fish chemoreceptor that detects one of these compounds. Using expression cloning in Xenopus oocytes coexpressing the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel, the beta-2 adrenergic receptor (beta(2)AR), and fractions of a zebrafish cDNA library, we isolated a cDNA clone encoding receptor activity-modifying protein (RAMP)-like triterpene glycoside receptor (RL-TGR), a novel coreceptor involved in signaling in response to triterpene glycosides. This coreceptor appears to be structurally and functionally related to RAMPs, a family of coreceptors that physically associate with and modify the activity of G protein-coupled receptors (GPCRs). In membranes from formoside-responsive oocytes, RL-TGR was immunoprecipitated in an apparent complex with beta(2)AR. In HEK293 cells, coexpression of beta(2)AR induced the trafficking of RL-TGR from the cytoplasm to the plasma membrane. These results suggest that RL-TGR in the predatory fish physically associates with the beta(2)AR or another, more physiologically relevant GPCR and modifies its pharmacology to respond to triterpene glycosides found in sponges that serve as a potential food source for the fish. RL-TGR forms a coreceptor that responds to a chemical defense compound in the marine environment, and its discovery might lead the way to the identification of other receptors that mediate chemical defense signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pawlik JR. Marine invertebrate chemical defenses. Chem Rev. 1993;93:1911–1922.
-
- Paul VJ, Ritson-Williams R. Marine chemical ecology. Nat Prod Rep. 2008;25:662–695. - PubMed
-
- Derby CD, Sorensen PW. Neural processing, perception, and behavioral responses to natural chemical stimuli by fish and crustaceans. J Chem Ecol. 2008;34:898–914. - PubMed
-
- Sheybani A, Nusnbaum M, Caprio J, Derby CD. Responses of the sea catfish Ariopsis felis to chemical defenses from the sea hare Aplysia californica. J Exp Mar Biol Ecol. 2009;368:153–160.
-
- Bickmeyer U. Bromoageliferin and dibromoageliferin, secondary metabolites from the marine sponge Agelas conifera, inhibit voltage-operated, but not store-operated, calcium entry in PC12 cells. Toxicon. 2005;45:627–632. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

