NIP/DuoxA is essential for Drosophila embryonic development and regulates oxidative stress response
- PMID: 20567495
- PMCID: PMC2878171
- DOI: 10.7150/ijbs.6.252
NIP/DuoxA is essential for Drosophila embryonic development and regulates oxidative stress response
Abstract
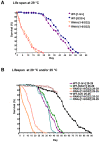
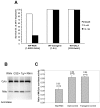
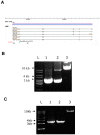
NIP/DuoxA, originally cloned as a protein capable of binding to the cell fate determinant Numb in Drosophila, was recently identified as a modulator of reactive oxygen species (ROS) production in mammalian systems. Despite biochemical and cellular studies that link NIP/DuoxA to the generation of ROS through the dual oxidase (Duox) enzyme, the in vivo function of NIP/DuoxA has not been characterized to date. Here we report a genetic and functional characterization of nip in Drosophila melanogaster. We show that nip is essential for Drosophila development as nip null mutants die at the 1(st) larval instar. Expression of UAS-nip, but not UAS-Duox, rescued the lethality. To understand the function of nip beyond the early larval stage, we generated GAL4 inducible UAS-RNAi transgenes. da(G32)-GAL4 driven, ubiquitous RNAi-mediated silencing of nip led to profound abnormality in pre-adult development, crinkled wing and markedly reduced lifespan at 29 degrees C. Compared to wild type flies, da-GAL4 induced nip-RNAi transgenic flies exhibited significantly reduced ability to survive under oxidative stress and displayed impaired mitochondrial aconitase function. Our work provides in vivo evidence for a critical role for nip in the development and oxidative stress response in Drosophila.
Keywords: Numb Interacting protein; dual oxidase maturation factor; embryonic development; oxidative stress..
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures

References
-
- Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell. 1989;58(2):349–360. - PubMed
-
- Knoblich JA, Jan LY, Jan YN. Asymmetric segregation of Numb and Prospero during cell division. Nature. 1995;377(6550):624–627. - PubMed
-
- Knoblich JA. Asymmetric cell division during animal development. Nat Rev Mol Cell Biol. 2001;2(1):11–20. - PubMed
-
- Li SC, Zwahlen C, Vincent SJ, McGlade CJ, Kay LE, Pawson T, Forman-Kay JD. Structure of a Numb PTB domain-peptide complex suggests a basis for diverse binding specificity. Nat Struct Biol. 1998;5(12):1075–1083. - PubMed
-
- Schlessinger J, Lemmon MA. SH2 and PTB domains in tyrosine kinase signaling. Sci STKE. 2003;2003(191):RE12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

