Melatonin plays a protective role in postburn rodent gut pathophysiology
- PMID: 20567497
- PMCID: PMC2878173
- DOI: 10.7150/ijbs.6.282
Melatonin plays a protective role in postburn rodent gut pathophysiology
Abstract
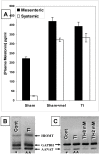
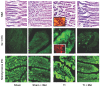
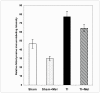
Melatonin is a possible protective agent in postburn gut pathophysiological dynamics. We investigated the role of endogenously-produced versus exogenously-administered melatonin in a major thermal injury rat model with well-characterized gut inflammatory complications. Our rationale is that understanding in vivo melatonin mechanisms in control and inflamed tissues will improve our understanding of its potential as a safe anti-inflammatory/antioxidant therapeutic alternative. Towards this end, we tested the hypothesis that the gut is both a source and a target for melatonin and that mesenteric melatonin plays an anti-inflammatory role following major thermal injury in rats with 3rd degree hot water scald over 30% TBSA. Our methods for assessing the gut as a source of melatonin included plasma melatonin ELISA measurements in systemic and mesenteric circulation as well as rtPCR measurement of jejunum and terminal ileum expression of the melatonin synthesizing enzymes arylalkylamine N-acetyltransferase (AA-NAT) and 5-hydroxyindole-O-methyltransferase (HIOMT) in sham versus day-3 postburn rats. Our melatonin ELISA results revealed that mesenteric circulation has much higher melatonin than systemic circulation and that both mesenteric and systemic melatonin levels are increased three days following major thermal injury. Our rtPCR results complemented the ELISA data in showing that the melatonin synthesizing enzymes AA-NAT and HIOMT are expressed in the ileum and jejunum and that this expression is increased three days following major thermal injury. Interestingly, the rtPCR data also revealed negative feedback by melatonin as exogenous melatonin supplementation at a dose of 7.43 mg (32 micromole/kg), but not 1.86 mg/kg (8 micromole/kg) drastically suppressed AA-NAT mRNA expression. Our methods also included an assessment of the gut as a target for melatonin utilizing computerized immunohistochemical measurements to quantify the effects of exogenous melatonin supplementation on postburn gut mucosa barrier inflammatory profiles. Here, our results revealed that daily postburn intraperitoneal melatonin administration at a dose of 1.86 mg/kg (8 micromole/kg) significantly suppressed both neutrophil infiltration and tyrosine nitrosylation as revealed by Gr-1 and nitrotyrosine immunohistochemistry, respectively. In conclusion, our results provide support for high mesenteric melatonin levels and dynamic de novo gut melatonin production, both of which increase endogenously in response to major thermal injury, but appear to fall short of abrogating the excessive postburn hyper-inflammation. Moreover, supplementation by exogenous melatonin significantly suppresses gut inflammation, thus confirming that melatonin is protective against postburn inflammation.
Keywords: AA-NAT; ELISA; GR1.; HIOMT; Melatonin; burn; extravasation; gut barrier; ileum; immunohistochemistry; inflammation; jejunum; mesenteric; neutrophils; nitrotyrosine; rtPCR; sepsis.
Conflict of interest statement
Conflict of Interest: The authors declare that they do not have any conflict of interest in this study.
Figures

References
-
- Al-Ghoul WM, Khan M, Fazal N. et al.Mechanisms of postburn intestinal barrier dysfunction in the rat: roles of epithelial cell renewal, E-cadherin, and neutrophil extravasation. Crit Care Med. 2004;32(8):1730–9. - PubMed
-
- Fazal N, Raziuddin S, Khan M. et al.Antigen presenting cells (APCs) from thermally injured and/or septic rats modulate CD4+ T cell responses of naive rat. Biochim Biophys Acta. 2006;1762(1):46–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

