Neural processing of short-term recurrence in songbird vocal communication
- PMID: 20567499
- PMCID: PMC2886052
- DOI: 10.1371/journal.pone.0011129
Neural processing of short-term recurrence in songbird vocal communication
Abstract
Background: Many situations involving animal communication are dominated by recurring, stereotyped signals. How do receivers optimally distinguish between frequently recurring signals and novel ones? Cortical auditory systems are known to be pre-attentively sensitive to short-term delivery statistics of artificial stimuli, but it is unknown if this phenomenon extends to the level of behaviorally relevant delivery patterns, such as those used during communication.
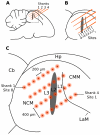
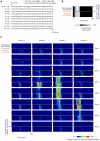
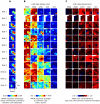
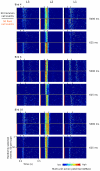
Methodology/principal findings: We recorded and analyzed complete auditory scenes of spontaneously communicating zebra finch (Taeniopygia guttata) pairs over a week-long period, and show that they can produce tens of thousands of short-range contact calls per day. Individual calls recur at time scales (median interval 1.5 s) matching those at which mammalian sensory systems are sensitive to recent stimulus history. Next, we presented to anesthetized birds sequences of frequently recurring calls interspersed with rare ones, and recorded, in parallel, action and local field potential responses in the medio-caudal auditory forebrain at 32 unique sites. Variation in call recurrence rate over natural ranges leads to widespread and significant modulation in strength of neural responses. Such modulation is highly call-specific in secondary auditory areas, but not in the main thalamo-recipient, primary auditory area.
Conclusions/significance: Our results support the hypothesis that pre-attentive neural sensitivity to short-term stimulus recurrence is involved in the analysis of auditory scenes at the level of delivery patterns of meaningful sounds. This may enable birds to efficiently and automatically distinguish frequently recurring vocalizations from other events in their auditory scene.
Conflict of interest statement
Figures




References
-
- Barlow H. Possible principles underlying the transformations of sensory messages. In: Rosenblith W, editor. Sensory Communication. Cambridge, Massachusetts: MIT Press; 1961. pp. 182–192.
-
- Müller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science. 1999;285:1405–1408. - PubMed
-
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–398. - PubMed
-
- Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. ‘Primitive intelligence’ in the auditory cortex. Trends Neurosci. 2001;24:283–288. - PubMed
-
- Nelken I, Ulanovsky N. Mismatch negativity and stimulus-specific adaptation in animal models. J Psychophysiol. 2007;21:214–223.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

