Comparison of the pharmacodynamic profiles of a biosimilar filgrastim and Amgen filgrastim: results from a randomized, phase I trial
- PMID: 20567823
- PMCID: PMC2924967
- DOI: 10.1007/s00277-010-0973-6
Comparison of the pharmacodynamic profiles of a biosimilar filgrastim and Amgen filgrastim: results from a randomized, phase I trial
Abstract
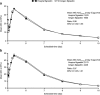
Further to the patent expiry of Neupogen (Amgen filgrastim), Hospira has developed a biosimilar filgrastim (Nivestim) that may offer a clinically effective alternative for multiple hematologic and oncologic indications. Here results are reported from a phase I trial, primarily designed to compare the pharmacodynamic profiles of Hospira filgrastim and Amgen filgrastim. A phase I, single-center, double-blind, randomized trial was undertaken to demonstrate equivalence of the pharmacodynamic characteristics of Hospira filgrastim and Amgen filgrastim. Fifty healthy volunteers were randomized to receive 5 or 10 microg/kg dosing, before further randomization to treatment sequence. All volunteers received five daily subcutaneous doses of Hospira filgrastim or Neupogen, with subsequent crossover to the alternative treatment. Bioequivalence was evaluated by analysis of variance; if the estimated 90% confidence intervals (CIs) for the ratio of 'test' to 'reference' treatment means were within the conventional equivalence limits of 0.80-1.25, then bioequivalence was concluded. Forty-eight volunteers completed the study. Geometric mean absolute neutrophil count area under the curve from time 0 to the last time point at day 5 (primary endpoint) was comparable in volunteers given Hospira filgrastim or Amgen filgrastim at 5 microg/kg (ratio of means, 0.98; 90% CI, 0.92-1.05) or 10 microg/kg (ratio, 0.97; 90% CI, 0.93-1.01); 90% CIs were within the predefined range necessary to demonstrate bioequivalence. Hospira filgrastim was well tolerated with no additional safety concerns over Amgen filgrastim. Hospira filgrastim is bioequivalent with Amgen filgrastim with regard to its pharmacodynamic characteristics.
Figures

Similar articles
-
Pharmacokinetic profiles of a biosimilar filgrastim and Amgen filgrastim: results from a randomized, phase I trial.Ann Hematol. 2010 Sep;89(9):927-33. doi: 10.1007/s00277-010-0961-x. Epub 2010 Apr 29. Ann Hematol. 2010. PMID: 20428872 Free PMC article. Clinical Trial.
-
Pharmacokinetic and pharmacodynamic profile of new biosimilar filgrastim XM02 equivalent to marketed filgrastim Neupogen: single-blind, randomized, crossover trial.BioDrugs. 2009;23(1):43-51. doi: 10.2165/00063030-200923010-00005. BioDrugs. 2009. PMID: 19344191 Clinical Trial.
-
Comparison of the physicochemical properties of a biosimilar filgrastim with those of reference filgrastim.Biologicals. 2010 Sep;38(5):557-66. doi: 10.1016/j.biologicals.2010.05.002. Epub 2010 Jul 16. Biologicals. 2010. PMID: 20637652
-
Comparing the effectiveness of biosimilar filgrastim (Nivestim®) versus original filgrastim (Neupogen®) for stem cell mobilization in adult and pediatric healthy donors.J Oncol Pharm Pract. 2023 Jun 26:10781552231171829. doi: 10.1177/10781552231171829. Online ahead of print. J Oncol Pharm Pract. 2023. PMID: 37357617 Review.
-
Pegfilgrastim: using pegylation technology to improve neutropenia support in cancer patients.Anticancer Drugs. 2003 Apr;14(4):259-64. doi: 10.1097/00001813-200304000-00002. Anticancer Drugs. 2003. PMID: 12679729 Review.
Cited by
-
Mobilization of Hematopoietic Stem Cells into Peripheral Blood for Autologous Transplantation Seems Less Efficacious in Poor Mobilizers with the Use of a Biosimilar of Filgrastim and Plerixafor: A Retrospective Comparative Analysis.Oncol Ther. 2020 Dec;8(2):311-324. doi: 10.1007/s40487-020-00115-3. Epub 2020 May 14. Oncol Ther. 2020. PMID: 32700041 Free PMC article.
-
Study design: two long-term observational studies of the biosimilar filgrastim Nivestim™ (Hospira filgrastim) in the treatment and prevention of chemotherapy-induced neutropenia.BMC Cancer. 2013 Nov 16;13:547. doi: 10.1186/1471-2407-13-547. BMC Cancer. 2013. PMID: 24237790 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Nivestim Versus Neupogen for Mobilization of Peripheral Blood Stem Cells for Autologous Stem Cell Transplantation.Sci Rep. 2019 Dec 27;9(1):19938. doi: 10.1038/s41598-019-56477-w. Sci Rep. 2019. PMID: 31882793 Free PMC article.
-
Characterization and comparison of commercially available TNF receptor 2-Fc fusion protein products.MAbs. 2012 Nov-Dec;4(6):761-74. doi: 10.4161/mabs.22276. Epub 2012 Oct 2. MAbs. 2012. PMID: 23032066 Free PMC article.
-
Trial Design and Statistical Considerations on the Assessment of Pharmacodynamic Similarity.AAPS J. 2019 Apr 3;21(3):47. doi: 10.1208/s12248-019-0321-2. AAPS J. 2019. PMID: 30945035
References
-
- Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M, Kearney N, Lyman GH, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–2453. doi: 10.1016/j.ejca.2006.05.002. - DOI - PubMed
-
- Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88:1907–1929. - PubMed
-
- Duhrsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988;72:2074–2081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

