Homocysteine is transported by the microvillous plasma membrane of human placenta
- PMID: 20567909
- PMCID: PMC2966547
- DOI: 10.1007/s10545-010-9141-3
Homocysteine is transported by the microvillous plasma membrane of human placenta
Abstract
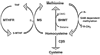
Elevated maternal plasma concentrations of homocysteine (Hcy) are associated with pregnancy complications and adverse neonatal outcomes. The postulate that we wish to advance here is that placental transport of Hcy, by competing with endogenous amino acids for transporter activity, may account for some of the damaging impacts of Hcy on placental metabolism and function as well as fetal development. In this article, we provide an overview of some recent studies characterising the transport mechanisms for Hcy across the microvillous plasma membrane (MVM) of the syncytiotrophoblast, the transporting epithelium of human placenta. Three Hcy transport systems have been identified, systems L, A and y(+)L. This was accomplished using a strategy of competitive inhibition to investigate the effects of Hcy on the uptake of well-characterised radiolabelled substrates for each transport system into isolated MVM vesicles. The reverse experiments were also performed, examining the effects of model substrates on [³⁵S]L-Hcy uptake. This article describes the evidence for systems L, A and y(+)L involvement in placental Hcy transport and discusses the physiological implications of these findings with respect to placental function and fetal development.
Conflict of interest statement
Conflict of interests: None to declare.
Figures


Similar articles
-
Homocysteine transport by systems L, A and y+L across the microvillous plasma membrane of human placenta.J Physiol. 2009 Aug 15;587(Pt 16):4001-13. doi: 10.1113/jphysiol.2009.173393. Epub 2009 Jun 29. J Physiol. 2009. PMID: 19564394 Free PMC article.
-
Expression of folate transporters in human placenta and implications for homocysteine metabolism.Placenta. 2010 Feb;31(2):134-43. doi: 10.1016/j.placenta.2009.11.017. Epub 2009 Dec 28. Placenta. 2010. PMID: 20036773
-
L-serine uptake by human placental microvillous membrane vesicles.Placenta. 2007 May-Jun;28(5-6):445-52. doi: 10.1016/j.placenta.2006.06.014. Epub 2006 Aug 10. Placenta. 2007. PMID: 16904742
-
The mechanisms and regulation of placental amino acid transport to the human foetus.J Neuroendocrinol. 2008 Apr;20(4):419-26. doi: 10.1111/j.1365-2826.2008.01662.x. Epub 2008 Feb 8. J Neuroendocrinol. 2008. PMID: 18266945 Review.
-
Placental amino acid transport.Am J Physiol. 1995 Jun;268(6 Pt 1):C1321-31. doi: 10.1152/ajpcell.1995.268.6.C1321. Am J Physiol. 1995. PMID: 7611349 Review.
Cited by
-
Effects of Dietary Choline Levels During Pregnancy on Reproductive Performance, Plasma Metabolome and Gut Microbiota of Sows.Front Vet Sci. 2022 Jan 24;8:771228. doi: 10.3389/fvets.2021.771228. eCollection 2021. Front Vet Sci. 2022. PMID: 35141305 Free PMC article.
-
Maternal Hyperhomocysteinemia Induces Neuroinflammation and Neuronal Death in the Rat Offspring Cortex.Neurotox Res. 2020 Aug;38(2):408-420. doi: 10.1007/s12640-020-00233-w. Epub 2020 Jun 5. Neurotox Res. 2020. PMID: 32504390
-
Biochemical Hazards during Three Phases of Assisted Reproductive Technology: Repercussions Associated with Epigenesis and Imprinting.Int J Mol Sci. 2022 Aug 10;23(16):8916. doi: 10.3390/ijms23168916. Int J Mol Sci. 2022. PMID: 36012172 Free PMC article. Review.
-
Longitudinal Analysis of One-Carbon Metabolism-Related Metabolites in Maternal and Cord Blood of Japanese Pregnant Women.Nutrients. 2024 Jun 4;16(11):1765. doi: 10.3390/nu16111765. Nutrients. 2024. PMID: 38892698 Free PMC article.
-
Methionine, homocysteine, one carbon metabolism and fetal growth.Rev Endocr Metab Disord. 2012 Jun;13(2):109-19. doi: 10.1007/s11154-012-9215-7. Rev Endocr Metab Disord. 2012. PMID: 22418620 Review.
References
-
- Ayuk PT, Sibley CP, Donnai P, et al. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278:C1162–C1171. - PubMed
-
- Bodoy S, Martin L, Zorzano A, et al. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. - PubMed
-
- Brunini TM, Yaqoob MM, Novaes Malagris LE, et al. Increased nitric oxide synthesis in uraemic platelets is dependent on L-arginine transport via system y(+)L. Pflugers Arch. 2003;445:547–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

