Enhanced protein repair and recycling are not correlated with longevity in 15 vertebrate endotherm species
- PMID: 20567926
- PMCID: PMC3063641
- DOI: 10.1007/s11357-010-9157-5
Enhanced protein repair and recycling are not correlated with longevity in 15 vertebrate endotherm species
Abstract
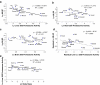
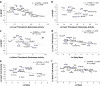
Previous studies have shown that longevity is associated with enhanced cellular stress resistance. This observation supports the disposable soma theory of aging, which suggests that the investment made in cellular maintenance will be proportional to selective pressures to extend lifespan. Maintenance of protein homeostasis is a critical component of cellular maintenance and stress resistance. To test the hypothesis that enhanced protein repair and recycling activities underlie longevity, we measured the activities of the 20S/26S proteasome and two protein repair enzymes in liver, heart and brain tissues of 15 different mammalian and avian species with maximum lifespans (MLSP) ranging from 3 to 30 years. The data set included Snell dwarf mice, in which lifespan is increased by ∼50% compared to their normal littermates. None of these activities in any of the three tissues correlated positively with MLSP. In liver, 20S/26S proteasome and thioredoxin reductase (TrxR) activities correlated negatively with body mass. In brain tissue, TrxR was also negatively correlated with body mass. Glutaredoxin (Grx) activity in brain was negatively correlated with MLSP and this correlation remained after residual analysis to remove the effects of body mass, but was lost when the data were analysed using Felsenstein's independent contrasts. Snell dwarf mice had marginally lower 20S proteasome, TrxR and Grx activities than normal controls in brain, but not heart tissue. Thus, increased longevity is not associated with increased protein repair or proteasomal degradation capacities in vertebrate endotherms.
Figures




Similar articles
-
Antioxidant enzyme activities are not broadly correlated with longevity in 14 vertebrate endotherm species.Age (Dordr). 2010 Jun;32(2):255-70. doi: 10.1007/s11357-010-9131-2. Epub 2010 Jan 27. Age (Dordr). 2010. PMID: 20431992 Free PMC article.
-
Determinants of rodent longevity in the chaperone-protein degradation network.Cell Stress Chaperones. 2016 May;21(3):453-66. doi: 10.1007/s12192-016-0672-x. Epub 2016 Feb 19. Cell Stress Chaperones. 2016. PMID: 26894765 Free PMC article.
-
Higher levels of heat shock proteins in longer-lived mammals and birds.Mech Ageing Dev. 2011 Jun-Jul;132(6-7):287-97. doi: 10.1016/j.mad.2011.06.002. Epub 2011 Jun 15. Mech Ageing Dev. 2011. PMID: 21703294
-
Degradation of oxidized proteins by the proteasome: Distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways.Mol Aspects Med. 2016 Aug;50:41-55. doi: 10.1016/j.mam.2016.05.001. Epub 2016 May 4. Mol Aspects Med. 2016. PMID: 27155164 Free PMC article. Review.
-
Protein homeostasis and aging: role of ubiquitin protein ligases.Neurochem Int. 2012 Apr;60(5):443-7. doi: 10.1016/j.neuint.2012.02.009. Epub 2012 Feb 15. Neurochem Int. 2012. PMID: 22353631 Review.
Cited by
-
Testing predictions of the oxidative stress hypothesis of aging using a novel invertebrate model of longevity: the giant clam (Tridacna derasa).J Gerontol A Biol Sci Med Sci. 2013 Apr;68(4):359-67. doi: 10.1093/gerona/gls159. Epub 2012 Aug 17. J Gerontol A Biol Sci Med Sci. 2013. PMID: 22904097 Free PMC article.
-
Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal.J Gerontol A Biol Sci Med Sci. 2011 Jul;66(7):741-50. doi: 10.1093/gerona/glr044. Epub 2011 Apr 12. J Gerontol A Biol Sci Med Sci. 2011. PMID: 21486920 Free PMC article.
-
A comparative cellular and molecular biology of longevity database.Age (Dordr). 2013 Oct;35(5):1937-47. doi: 10.1007/s11357-012-9458-y. Epub 2012 Jul 27. Age (Dordr). 2013. PMID: 22836712 Free PMC article. Review.
-
Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS).Dose Response. 2014 Jan 31;12(2):288-341. doi: 10.2203/dose-response.13-035.Ristow. eCollection 2014 May. Dose Response. 2014. PMID: 24910588 Free PMC article.
-
Mitochondrial DNA damage and animal longevity: insights from comparative studies.J Aging Res. 2011 Mar 2;2011:807108. doi: 10.4061/2011/807108. J Aging Res. 2011. PMID: 21423601 Free PMC article.
References
-
- Arner ESJ. Focus on mammalian thioredoxin reductases—important selenoproteins with versatile functions. Biochim Biophys Acta. 2009;1790:495–526. - PubMed
-
- Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. - PubMed
-
- Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226:73–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
