Epigenomic disruption: the effects of early developmental exposures
- PMID: 20568270
- PMCID: PMC2945443
- DOI: 10.1002/bdra.20685
Epigenomic disruption: the effects of early developmental exposures
Abstract
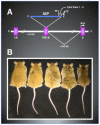
Through DNA methylation, histone modifications, and small regulatory RNAs the epigenome systematically controls gene expression during development, both in utero and throughout life. The epigenome is also a very reactive system; its labile nature allows it to sense and respond to environmental perturbations to ensure survival during fetal growth. This pliability can lead to aberrant epigenetic modifications that persist into later life and induce numerous disease states. Endocrine-disrupting compounds (EDCs) are ubiquitous chemicals that interfere with growth and development. Several EDCs also interfere with epigenetic programming. The investigation of the epigenotoxic effects of bisphenol A (BPA), an EDC used in the production of plastics and resins, has further raised concern over the impact of EDCs on the epigenome. Using the Agouti viable yellow (A(vy)) mouse model, dietary BPA exposure was shown to hypomethylate both the A(vy) and the Cabp(IAP) metastable epialleles. This hypomethylating effect was counteracted with dietary supplementation of methyl donors or genistein. These results are consistent with reports of BPA and other EDCs causing epigenetic effects. Epigenotoxicity could lead to numerous developmental, metabolic, and behavioral disorders in exposed populations. The heritable nature of epigenetic changes also increases the risk for transgenerational inheritance of phenotypes. Thus, epigenotoxicity must be considered when assessing these compounds for safety.
© 2010 Wiley-Liss, Inc.
Figures


References
-
- Baqir S, Smith L. Growth restricted in vitro culture conditions alter the imprinted gene expression patterns of mouse embryonic stem cells. Cloning Stem Cells. 2003;5(3):199–121. - PubMed
-
- Barker D, Eriksson J, Forsen T, Osmond C. Fetal origins of adult disease: Strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. - PubMed
-
- Barker D, Osmond C, Kajantie E, Eriksson J. Growth and chronic disease: findings in the Helsinki birth cohort. Annals of Human Biology. 2009;36(5):445–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

