Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis
- PMID: 20568301
- PMCID: PMC2932809
- DOI: 10.1002/hep.23783
Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis
Abstract
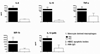
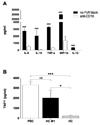
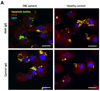
Our understanding of primary biliary cirrhosis (PBC) has been significantly enhanced by the rigorous dissection of the multilineage T and B cell response against the immunodominant mitochondrial autoantigen, the E2 component of the pyruvate dehydrogenase complex (PDC-E2). PDC-E2 is a ubiquitous protein present in mitochondria of nucleated cells. However, the damage of PBC is confined to small biliary epithelial cells (BECs). We have previously demonstrated that BECs translocate immunologically intact PDC-E2 to apoptotic bodies and create an apotope. To define the significance of this observation, we have studied the ability of biliary or control epithelial apotopes to induce cytokine secretion from mature monocyte-derived macrophages (MDMphis) from either patients with PBC or controls in the presence or absence of anti-mitochondrial antibodies (AMAs). We demonstrate that there is intense inflammatory cytokine production in the presence of the unique triad of BEC apotopes, macrophages from patients with PBC, and AMAs. The cytokine secretion is inhibited by anti-CD16 and is not due to differences in apotope uptake. Moreover, MDMphis from PBC patients cultured with BEC apoptotic bodies in the presence of AMAs markedly increase tumor necrosis factor-related apoptosis-inducing ligand expression.
Conclusion: These results provide a mechanism for the biliary specificity of PBC, the recurrence of disease after liver transplantation, and the success of ursodiol in treatment. They further emphasize the critical role of the innate immune system in the perpetuation of this autoimmune disease.
Conflict of interest statement
Figures





References
-
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. - PubMed
-
- Kawano A, Shimoda S, Kamihira T, Ishikawa F, Niiro H, Soejima Y, Taketomi A, et al. Peripheral tolerance and the qualitative characteristics of autoreactive T cell clones in primary biliary cirrhosis. J Immunol. 2007;179:3315–3324. - PubMed
-
- Shimoda S, Miyakawa H, Nakamura M, Ishibashi H, Kikuchi K, Kita H, Niiro H, et al. CD4 T-cell autoreactivity to the mitochondrial autoantigen PDC-E2 in AMA-negative primary biliary cirrhosis. J Autoimmun. 2008;31:110–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
