Mechanistic investigation of the inhibition of Abeta42 assembly and neurotoxicity by Abeta42 C-terminal fragments
- PMID: 20568734
- PMCID: PMC2912417
- DOI: 10.1021/bi100773g
Mechanistic investigation of the inhibition of Abeta42 assembly and neurotoxicity by Abeta42 C-terminal fragments
Abstract
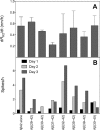
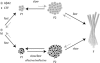
Oligomeric forms of amyloid beta-protein (Abeta) are key neurotoxins in Alzheimer's disease (AD). Previously, we found that C-terminal fragments (CTFs) of Abeta42 interfered with assembly of full-length Abeta42 and inhibited Abeta42-induced toxicity. To decipher the mechanism(s) by which CTFs affect Abeta42 assembly and neurotoxicity, here, we investigated the interaction between Abeta42 and CTFs using photoinduced cross-linking and dynamic light scattering. The results demonstrate that distinct parameters control CTF inhibition of Abeta42 assembly and Abeta42-induced toxicity. Inhibition of Abeta42-induced toxicity was found to correlate with stabilization of oligomers with a hydrodynamic radius (R(H)) of 8-12 nm and attenuation of formation of oligomers with an R(H) of 20-60 nm. In contrast, inhibition of Abeta42 paranucleus formation correlated with CTF solubility and the degree to which CTFs formed amyloid fibrils themselves but did not correlate with inhibition of Abeta42-induced toxicity. Our findings provide important insight into the mechanisms by which different CTFs inhibit the toxic effect of Abeta42 and suggest that stabilization of nontoxic Abeta42 oligomers is a promising strategy for designing inhibitors of Abeta42 neurotoxicity.
Figures


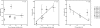
References
-
- Prince M, Jackson J, editors. Alzheimer's Disease International World Alzheimer Report 2009. Alzheimer's Disease International; London: 2009.
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Ferreira ST, Vieira MN, De Felice FG. Soluble protein oligomers as emerging toxins in Alzheimer's and other amyloid diseases. IUBMB Life. 2007;59:332–345. - PubMed
-
- Dahlgren KN, Manelli AM, Stine WB, Jr., Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J. Biol. Chem. 2002;277:32046–32053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

