Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length
- PMID: 20569421
- PMCID: PMC2905357
- DOI: 10.1186/1465-9921-11-84
Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length
Abstract
Background: Poor gene transfer efficiency has been a major problem in developing an effective gene therapy for cystic fibrosis (CF) airway disease. Lysophosphatidylcholine (LPC), a natural airway surfactant, can enhance viral gene transfer in animal models. We examined the electrophysiological and physical effect of airway pre-treatment with variants of LPC on lentiviral (LV) vector gene transfer efficiency in murine nasal airways in vivo.
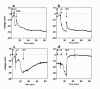
Methods: Gene transfer was assessed after 1 week following nasal instillations of a VSV-G pseudotype LV vector pre-treated with a low and high dose of LPC variants. The electrophysiological effects of a range of LPC variants were assessed by nasal transepithelial potential difference measurements (TPD) to determine tight junction permeability. Any physical changes to the epithelium from administration of the LPC variants were noted by histological methods in airway tissue harvested after 1 hour.
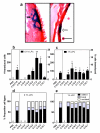
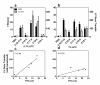
Results: Gene transduction was significantly greater compared to control (PBS) for our standard LPC (palmitoyl/stearoyl mixture) treatment and for the majority of the other LPC variants with longer acyl chain lengths. The LPC variant heptadecanoyl also produced significantly greater LV gene transfer compared to our standard LPC mixture. LV gene transfer and the transepithelial depolarization produced by the 0.1% LPC variants at 1 hour were strongly correlated (r2 = 0.94), but at the 1% concentration the correlation was less strong (r2 = 0.59). LPC variants that displayed minor to moderate levels of disruption to the airway epithelium were clearly associated with higher LV gene transfer.
Conclusions: These findings show the LPC variants effect on airway barrier function and their correlation to the effectiveness of gene expression. The enhanced expression produced by a number of LPC variants should provide new options for preclinical development of efficient airway gene transfer techniques.
Figures

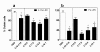

Similar articles
-
Effective viral-mediated lung gene therapy: is airway surface preparation necessary?Gene Ther. 2023 Jun;30(6):469-477. doi: 10.1038/s41434-022-00332-7. Epub 2022 Mar 29. Gene Ther. 2023. PMID: 35351979 Free PMC article. Review.
-
Long-term therapeutic and reporter gene expression in lentiviral vector treated cystic fibrosis mice.J Gene Med. 2014 Sep-Oct;16(9-10):291-9. doi: 10.1002/jgm.2778. J Gene Med. 2014. PMID: 25130650
-
Gene therapy for Cystic Fibrosis: Improved delivery techniques and conditioning with lysophosphatidylcholine enhance lentiviral gene transfer in mouse lung airways.Exp Lung Res. 2017 Nov-Dec;43(9-10):426-433. doi: 10.1080/01902148.2017.1395931. Epub 2017 Dec 13. Exp Lung Res. 2017. PMID: 29236544
-
Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer.Hum Gene Ther. 2002 Nov 1;13(16):1961-70. doi: 10.1089/10430340260355365. Hum Gene Ther. 2002. PMID: 12427306
-
Lentivirus-mediated gene transfer to the respiratory epithelium: a promising approach to gene therapy of cystic fibrosis.Gene Ther. 2004 Oct;11 Suppl 1:S67-75. doi: 10.1038/sj.gt.3302372. Gene Ther. 2004. PMID: 15454960 Review.
Cited by
-
The Y498T499-SARS-CoV-2 spike (S) protein interacts poorly with rat ACE2 and does not affect the rat lung.Access Microbiol. 2024 Sep 27;6(9):000839.v3. doi: 10.1099/acmi.0.000839.v3. eCollection 2024. Access Microbiol. 2024. PMID: 39346684 Free PMC article.
-
Advances in cell and gene-based therapies for cystic fibrosis lung disease.Mol Ther. 2012 Jun;20(6):1108-15. doi: 10.1038/mt.2012.32. Epub 2012 Feb 28. Mol Ther. 2012. PMID: 22371844 Free PMC article. Review.
-
Effective viral-mediated lung gene therapy: is airway surface preparation necessary?Gene Ther. 2023 Jun;30(6):469-477. doi: 10.1038/s41434-022-00332-7. Epub 2022 Mar 29. Gene Ther. 2023. PMID: 35351979 Free PMC article. Review.
-
Cystic Fibrosis Gene Therapy in the UK and Elsewhere.Hum Gene Ther. 2015 May;26(5):266-75. doi: 10.1089/hum.2015.027. Hum Gene Ther. 2015. PMID: 25838137 Free PMC article. Review.
-
Lentiviral-mediated phenotypic correction of cystic fibrosis pigs.JCI Insight. 2016 Sep 8;1(14):e88730. doi: 10.1172/jci.insight.88730. JCI Insight. 2016. PMID: 27656681 Free PMC article.
References
-
- Rosenecker J, Huth S, Rudolph C. Gene therapy for cystic fibrosis lung disease: current status and future perspectives. Curr Opin Mol Ther. 2006;8:439–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

