A dietary supplement to improve the quality of sleep: a randomized placebo controlled trial
- PMID: 20569455
- PMCID: PMC2901361
- DOI: 10.1186/1472-6882-10-29
A dietary supplement to improve the quality of sleep: a randomized placebo controlled trial
Abstract
Background: To evaluate the effect of a dietary supplement containing polyunsaturated fatty acids, in association with Humulus lupulus extract, on the quality of sleep using the Leeds sleep evaluation questionnaire (LSEQ) in subjects with moderate to severe sleep disorders.
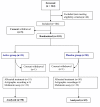
Methods: Randomized placebo-controlled trial, in a Population-based setting. Participants were adult patients 25 to 65 years old with a chronic primary insomnia who volunteered for the study. The tested intervention consisted of two soft gelatine capsules per day, containing either the dietary supplement (active group) or olive oil (placebo group) for a month. Subjects could also volunteer for two ancillary studies on melatonin and actigraphy. Evaluation criteria included i) perception of the quality of sleep at the end of treatment using the LSEQ questionnaire, ii) sleep efficiency measured by one-week actigraphic movement measurement performed before and during the treatment in a subsample of subjects, iii) night melatonin and 6 sulfatoxymelatonin (aMT6S) urine rates in a subsample of subjects.
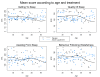
Results: The average of Leeds score was similar in both groups (p = 0.95). A marked improvement in the quality of sleep was observed in both placebo (62%) and active (65%) group (p = 0.52). The evolution of urinary melatonin, aMT6S, and of the Mel/aMT6S ratio showed no differences between the two groups. Sleep efficiency, as measured by actigraphy, improved similarly in both groups during the treatment period, from 72% to 76% and 75% in the active and placebo group respectively (p = 0.91).
Conclusions: The dietary supplement had neither effect on the perceived quality of sleep, nor on the melatonin metabolism and sleep-wake cycle.
Trial registration: clinical trials.gov:NCT00484497.
Figures
Similar articles
-
Efficacy of melatonin with behavioural sleep-wake scheduling for delayed sleep-wake phase disorder: A double-blind, randomised clinical trial.PLoS Med. 2018 Jun 18;15(6):e1002587. doi: 10.1371/journal.pmed.1002587. eCollection 2018 Jun. PLoS Med. 2018. PMID: 29912983 Free PMC article. Clinical Trial.
-
Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality.Eur J Nutr. 2012 Dec;51(8):909-16. doi: 10.1007/s00394-011-0263-7. Epub 2011 Oct 30. Eur J Nutr. 2012. PMID: 22038497 Clinical Trial.
-
Dietary Supplementation with an Extract of Aloysia citrodora (Lemon verbena) Improves Sleep Quality in Healthy Subjects: A Randomized Double-Blind Controlled Study.Nutrients. 2024 May 18;16(10):1523. doi: 10.3390/nu16101523. Nutrients. 2024. PMID: 38794761 Free PMC article. Clinical Trial.
-
Influence of Dietary Sources of Melatonin on Sleep Quality: A Review.J Food Sci. 2020 Jan;85(1):5-13. doi: 10.1111/1750-3841.14952. Epub 2019 Dec 19. J Food Sci. 2020. PMID: 31856339
-
Cross-cultural validation of the Leeds sleep evaluation questionnaire (LSEQ) in insomnia patients.Hum Psychopharmacol. 2003 Dec;18(8):603-10. doi: 10.1002/hup.534. Hum Psychopharmacol. 2003. PMID: 14696019 Review.
Cited by
-
A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials Evaluating the Evidence Base of Melatonin, Light Exposure, Exercise, and Complementary and Alternative Medicine for Patients with Insomnia Disorder.J Clin Med. 2020 Jun 22;9(6):1949. doi: 10.3390/jcm9061949. J Clin Med. 2020. PMID: 32580450 Free PMC article. Review.
-
Associations of Dietary ω-3, ω-6 Fatty Acids Consumption with Sleep Disorders and Sleep Duration among Adults.Nutrients. 2021 Apr 27;13(5):1475. doi: 10.3390/nu13051475. Nutrients. 2021. PMID: 33925486 Free PMC article.
-
Cinnamon and Hop Extracts as Potential Immunomodulators for Severe COVID-19 Cases.Front Plant Sci. 2021 Feb 26;12:589783. doi: 10.3389/fpls.2021.589783. eCollection 2021. Front Plant Sci. 2021. PMID: 33719281 Free PMC article. No abstract available.
-
Omega-3 long-chain polyunsaturated fatty acid and sleep: a systematic review and meta-analysis of randomized controlled trials and longitudinal studies.Nutr Rev. 2021 Jul 7;79(8):847-868. doi: 10.1093/nutrit/nuaa103. Nutr Rev. 2021. PMID: 33382879 Free PMC article.
-
Effects of sleep-inducing juice on sleep quality and heart rate variability in adults with disturbed sleep.Nutr Res Pract. 2020 Dec;14(6):606-620. doi: 10.4162/nrp.2020.14.6.606. Epub 2020 Aug 5. Nutr Res Pract. 2020. PMID: 33282123 Free PMC article.
References
-
- Leger D. Public health and insomnia: economic impact. Sleep. 2000;23(Suppl 3):S69–76. - PubMed
-
- Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65(Suppl 8):13–9. - PubMed
-
- Buysse DJ. Clinical diagnoses in 216 insomnia patients using the International Classification of Sleep Disorders (ICSD), DSM-IV and ICD-10 categories: a report from the APA/NIMH DSM-IV Field Trial. Sleep. 1994;17(7):630–7. - PubMed
-
- Leger D, Levy E, Paillard M. The direct costs of insomnia in France. Sleep. 1999;22(Suppl 2):S394–401. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical