Ezrin promotes invasion and metastasis of pancreatic cancer cells
- PMID: 20569470
- PMCID: PMC2916894
- DOI: 10.1186/1479-5876-8-61
Ezrin promotes invasion and metastasis of pancreatic cancer cells
Abstract
Background: Pancreatic cancer has a high mortality rate because it is usually diagnosed when metastasis have already occurred (microscopic and gross disease). Ezrin plays important roles in cell motility, invasion and tumor progression, and it is especially crucial for metastasis. However, its function in pancreatic cancer remains elusive.
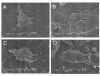
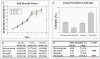
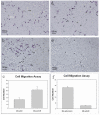
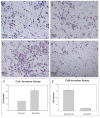
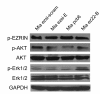
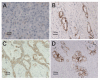
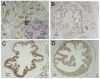
Methods and results: We found that ezrin overexpression promoted cell protrusion, microvillus formation, anchorage-independent growth, motility and invasion in a pancreatic cancer cell line, MiaPaCa-2, whereas ezrin silencing resulted in the opposite effects. Ezrin overexpression also increased the number of metastatic foci (6/8 vs. 1/8) in a spontaneous metastasis nude mouse model. Furthermore, ezrin overexpression activated Erk1/2 in MiaPaCa-2 cells, which might be partially related to the alteration of cell morphology and invasion. Immunohistochemical analysis showed that ezrin was overexpressed in pancreatic ductal adenocarcinoma (PDAC) (91.4%) and precancerous lesions, i.e. the tubular complexes in chronic pancreatitis (CP) and pancreatic intraepithelial neoplasm (PanIN) (85.7% and 97.1%, respectively), compared to normal pancreatic tissues (0%). Ezrin was also expressed in intercalated ducts adjacent to the adenocarcinoma, which has been considered to be the origin of ducts and acini, as well as the starting point of pancreatic ductal carcinoma development.
Conclusions: We propose that ezrin might play functional roles in modulating morphology, growth, motility and invasion of pancreatic cancer cells, and that the Erk1/2 pathway may be involved in these roles. Moreover, ezrin may participate in the early events of PDAC development and may promote its progression to the advanced stage.
Figures

Similar articles
-
Ezrin protein overexpression predicts the poor prognosis of pancreatic ductal adenocarcinomas.Exp Mol Pathol. 2015 Feb;98(1):1-6. doi: 10.1016/j.yexmp.2014.11.003. Epub 2014 Nov 5. Exp Mol Pathol. 2015. PMID: 25445504
-
[Effects of ezrin silencing on pancreatic cancer cell line Panc-1].Zhonghua Bing Li Xue Za Zhi. 2012 Dec;41(12):833-6. doi: 10.3760/cma.j.issn.0529-5807.2012.12.009. Zhonghua Bing Li Xue Za Zhi. 2012. PMID: 23324233 Chinese.
-
Aberrant expression of a disintegrin and metalloproteinase 17/tumor necrosis factor-alpha converting enzyme increases the malignant potential in human pancreatic ductal adenocarcinoma.Cancer Res. 2006 Sep 15;66(18):9045-53. doi: 10.1158/0008-5472.CAN-05-3287. Cancer Res. 2006. PMID: 16982746
-
Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis.Cells. 2023 Jun 14;12(12):1620. doi: 10.3390/cells12121620. Cells. 2023. PMID: 37371090 Free PMC article. Review.
-
Ezrin gone rogue in cancer progression and metastasis: An enticing therapeutic target.Biochim Biophys Acta Rev Cancer. 2022 Jul;1877(4):188753. doi: 10.1016/j.bbcan.2022.188753. Epub 2022 Jun 22. Biochim Biophys Acta Rev Cancer. 2022. PMID: 35752404 Review.
Cited by
-
Silence of ezrin modifies migration and actin cytoskeleton rearrangements and enhances chemosensitivity of lung cancer cells in vitro.Mol Cell Biochem. 2013 May;377(1-2):207-18. doi: 10.1007/s11010-013-1586-x. Epub 2013 Feb 22. Mol Cell Biochem. 2013. PMID: 23435957
-
Don't sugarcoat it: How glycocalyx composition influences cancer progression.J Cell Biol. 2020 Jan 6;219(1):e201910070. doi: 10.1083/jcb.201910070. J Cell Biol. 2020. PMID: 31874115 Free PMC article. Review.
-
The Mutant p53-Driven Secretome Has Oncogenic Functions in Pancreatic Ductal Adenocarcinoma Cells.Biomolecules. 2020 Jun 9;10(6):884. doi: 10.3390/biom10060884. Biomolecules. 2020. PMID: 32526853 Free PMC article.
-
12-O-Tetradecanoylphorbol-13-Acetate Induces Up-Regulated Transcription of Variant 1 but Not Variant 2 of VIL2 in Esophageal Squamous Cell Carcinoma Cells via ERK1/2/AP-1/Sp1 Signaling.PLoS One. 2015 Apr 27;10(4):e0124680. doi: 10.1371/journal.pone.0124680. eCollection 2015. PLoS One. 2015. PMID: 25915860 Free PMC article.
-
Ezrin Promotes Stem Cell Properties in Pancreatic Ductal Adenocarcinoma.Mol Cancer Res. 2019 Apr;17(4):929-936. doi: 10.1158/1541-7786.MCR-18-0367. Epub 2019 Jan 17. Mol Cancer Res. 2019. PMID: 30655325 Free PMC article.
References
-
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103(Pt 1):131–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

