Dopamine D1 receptor-mediated NMDA receptor insertion depends on Fyn but not Src kinase pathway in prefrontal cortical neurons
- PMID: 20569495
- PMCID: PMC2902469
- DOI: 10.1186/1756-6606-3-20
Dopamine D1 receptor-mediated NMDA receptor insertion depends on Fyn but not Src kinase pathway in prefrontal cortical neurons
Abstract
Background: Interactions between dopamine and glutamate in the prefrontal cortex are essential for cognitive functions such as working memory. Modulation of N-methyl-D-aspartic acid (NMDA) receptor functions by dopamine D1 receptor is believed to play a critical role in these functions. The aim of the work reported here is to explore the signaling pathway underlying D1 receptor-mediated trafficking of NMDA receptors in cultured rat prefrontal cortical neurons.
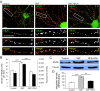
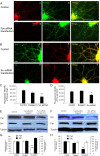
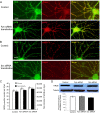
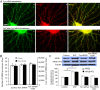
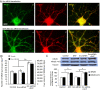
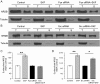
Results: Activation of D1 receptor by selective agonist SKF-81297 significantly increased the expression of NR2B subunits. This effect was completely blocked by small interfering RNA knockdown of Fyn, but not Src. Under control conditions, neither Fyn nor Src knockdown exhibited significant effect on basal NR2B expression. D1 stimulation significantly enhanced NR2B insertion into plasma membrane in cultured PFC neurons, a process obstructed by Fyn, but not Src, knockdown.
Conclusions: Dopamine D1 receptor-mediated increase of NMDA receptors is thus Fyn kinase dependent. Targeting this signaling pathway may be useful in treating drug addiction and schizophrenia.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

