74-week follow-up of safety of infliximab in patients with refractory rheumatoid arthritis
- PMID: 20569501
- PMCID: PMC2911915
- DOI: 10.1186/ar3058
74-week follow-up of safety of infliximab in patients with refractory rheumatoid arthritis
Abstract
Introduction: The objective was to describe the prevalence, types, and predictors of adverse events (AEs) in rheumatoid arthritis (RA) patients treated with infliximab and methotrexate in a daily clinical setting.
Methods: This was a prospective, multi-center, open-label, 74-week observational study in patients with active RA despite treatment with methotrexate and at least one other disease-modifying anti-rheumatic drug. Patients were treated with 3 mg/kg infliximab at weeks 0, 2, and 6 and then every 8 weeks. At weeks 0, 6, 26, 50, and 74, patients answered a health assessment questionnaire, a swollen joint count was made, and adverse events (AEs) occurring during the previous period were registered.
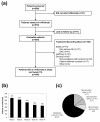
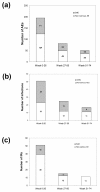
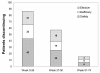
Results: Five hundred and seventy-five patients were treated with infliximab, of which 346 were still on infliximab at the study end, 158 discontinued treatment, and 71 were lost to follow-up. Reasons for discontinuation included safety (n=74), elective reasons (n=43), and inefficacy (n=41). Infusion reactions (n=33) and infections (n=20) were the most common AEs causing discontinuation and the most common AEs overall. There were four cases of tuberculosis, all of which occurred in patients negative at screening. Total AEs, serious AEs, and infusion reactions as well as discontinuations for AEs were most frequent during the first 26 weeks. Higher age was a predictor of serious adverse events (SAEs), infection, and discontinuation due to an SAE, but odds ratios were close to one.
Conclusions: AEs and discontinuations due to AEs occur most frequently during the first half year of infliximab treatment in refractory RA patients. The main reasons for discontinuing treatment are infections and infusion reactions. Tuberculosis and other infections remain an important concern in these patients.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

