CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination
- PMID: 20569695
- PMCID: PMC3856868
- DOI: 10.1016/j.stem.2010.04.002
CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination
Abstract
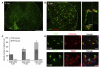
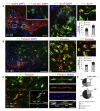
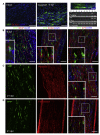
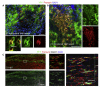
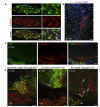
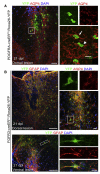
After central nervous system (CNS) demyelination-such as occurs during multiple sclerosis-there is often spontaneous regeneration of myelin sheaths, mainly by oligodendrocytes but also by Schwann cells. The origins of the remyelinating cells have not previously been established. We have used Cre-lox fate mapping in transgenic mice to show that PDGFRA/NG2-expressing glia, a distributed population of stem/progenitor cells in the adult CNS, produce the remyelinating oligodendrocytes and almost all of the Schwann cells in chemically induced demyelinated lesions. In contrast, the great majority of reactive astrocytes in the vicinity of the lesions are derived from preexisting FGFR3-expressing cells, likely to be astrocytes. These data resolve a long-running debate about the origins of the main players in CNS remyelination and reveal a surprising capacity of CNS precursors to generate Schwann cells, which normally develop from the embryonic neural crest and are restricted to the peripheral nervous system.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Arnett HA, Fancy SPJ, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. - PubMed
-
- Baron-Van Evercooren A, Avellana-Adalid V, Ben Younes-Chennoufi A, Gansmuller A, Nait-Oumesmar B, Vignais L. Cell-cell interactions during the migration of myelin-forming cells transplanted in the demyelinated spinal cord. Glia. 1996;16:147–164. - PubMed
-
- Blakemore WF. Remyelination by Schwann cells of axons demyelinated by intraspinal injection of 6-aminonicotinamide in the rat. J. Neurocytol. 1975;4:745–757. - PubMed
-
- Blakemore WF, Franklin RJM. Remyelination in experimental models of toxin-induced demyelination. Curr. Top. Microbiol. Immunol. 2008;318:193–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

