Wilms tumor chromatin profiles highlight stem cell properties and a renal developmental network
- PMID: 20569696
- PMCID: PMC2897075
- DOI: 10.1016/j.stem.2010.03.016
Wilms tumor chromatin profiles highlight stem cell properties and a renal developmental network
Abstract
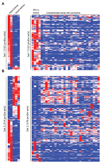
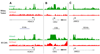
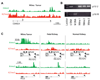
Wilms tumor is the most common pediatric kidney cancer. To identify transcriptional and epigenetic mechanisms that drive this disease, we compared genome-wide chromatin profiles of Wilms tumors, embryonic stem cells (ESCs), and normal kidney. Wilms tumors prominently exhibit large active chromatin domains previously observed in ESCs. In the cancer, these domains frequently correspond to genes that are critical for kidney development and expressed in the renal stem cell compartment. Wilms cells also express "embryonic" chromatin regulators and maintain stem cell-like p16 silencing. Finally, Wilms and ESCs both exhibit "bivalent" chromatin modifications at silent promoters that may be poised for activation. In Wilms tumor, bivalent promoters correlate to genes expressed in specific kidney compartments and point to a kidney-specific differentiation program arrested at an early-progenitor stage. We suggest that Wilms cells share a transcriptional and epigenetic landscape with a normal renal stem cell, which is inherently susceptible to transformation and may represent a cell of origin for this disease.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. - PubMed
-
- Bardeesy N, Falkoff D, Petruzzi MJ, Nowak N, Zabel B, Adam M, Aguiar MC, Grundy P, Shows T, Pelletier J. Anaplastic Wilms' tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat Genet. 1994;7:91–97. - PubMed
-
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. - PubMed
-
- Beckwith JB, Kiviat NB, Bonadio JF. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms' tumor. Pediatr Pathol. 1990;10:1–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

